Background: Non-alcoholic steatohepatitis (NASH) is a metabolic disorder of the liver, which may evolve to fibrosis or cirrhosis. Pentoxifylline (PTX) has been postulated to act as an antifibrogenic agent able to inhibit hepatic stellate cell proliferation and collagen synthesis in vitro. Short-term studies suggest beneficial effects of PTX in experimental models of NASH.
Aim: To study whether PTX can influence liver fibrogenesis in an animal model of NASH.
Methods: To induce NASH, a choline-deficient diet (CDD) was given to Sprague-Dawley rats for 8 weeks. Rats were allocated to two experimental groups one receiving PTX (9 mg/kg/day) in drinking water. Control rats received a choline-supplemented diet. Biochemical and histological evaluation of fatty liver was performed by conventional techniques. In addition, mRNA levels of Pro-collagen I and transforming growth factor beta-1 were assessed by semi-quantitative RT-PCR and stellate cell activation by α-actin immunofluorescence stain.
Results: After 8 weeks CDD induced a marked elevation of serum aminotransferases, a marked decrease in both hepatic and biliary glutathione and a severe fatty liver infiltration with mild histological inflammation and fibrosis. A significant increase in mRNA levels of both Pro-collagen I and TGFβ-1 was seen after CDD feeding. No differences were seen between rats receiving PTX and rats on CDD diet alone with regard to the biochemical, morphological or molecular alterations induced by the CDD.
Conclusion: PTX does influence neither liver injury nor early profibrogenic events in the CDD model of NASH.
Abbreviations:
PTX: Pentoxifylline
NAFLD: Nonalcoholic fatty liver disease
NASH: Non-alcoholic steatohepatitis
CDD: choline-deficient diet
CSD: Choline-sufficient diet
TGFβ: transforming growth factor beta
TNF-α: Tumor necrosis factor alpha
HSC: hepatic stellate cells
α-SMA: alpha-smooth muscle actin
hpf: high power field
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized health problem being the main cause of elevated serum liver enzymes among the general population and a cause of potentially progressive liver damage, posing patients at risk of advanced liver failure.1,2 NAFLD comprises a wide spectrum of histological abnormalities ranging from hepatic steatosis alone (fatty liver) to hepatic steatosis associated to various degrees of necro-inflammatory changes as well as pericellular and intralobular fibrosis.3 The term non-alcoholic steatohepatitis (NASH) is used when inflammation and fibrosis are present which is regarded as having the potential to progress to severe fibrosis or cirrhosis. Approximately 20% to 30% of patients with NAFLD have histological signs of NASH and are at higher risk of developing advanced fibrosis, cirrhosis and hepatocellular carcinoma.4-6
Knowledge about the factors responsible for the transition from uncomplicated NAFLD to progressively fibrosing steatohepatitis is limited.7 A conceptual model referred as the “2-hit hypothesis” has been proposed.8-10 In this model, the first hit is the accumulation of fatty acids and triglycerides in hepatocytes followed by a second hit, possibly of environmental or genetic origin, and development of an inflammatory process which ultimately may trigger a fibrogenic response resulting in significant collagen deposition. Thus, therapeutic strategies should be directed to influence both necroinflammatory activity and fibrogenesis. While the pathogenesis of necroinflammation in NASH is poorly understood, development of liver fibrosis is regarded, as in other liver diseases, as a stereotyped response to injury which mainly involves activation of hepatic stellate cells (HSC), the major collagen-producing cells in the injured liver. Similar to alcohol-induced liver disease, increased oxidative stress and cytokine-mediated injury are thought to be involved in the pathogenesis of NASH.7,11 Tumor necrosis factor alpha (TNF-α)-induced hepatocyte injury, which plays an important role in the development of alcohol-induced liver disease, has been also related to liver damage in NASH.12 In fact, elevated levels of serum TNF-α and increased hepatic TNF-α expression have been described in patients with NASH.13 Thus, manipulation of this cytokine may represent a novel therapeutic target NASH.
Pentoxifylline (PTX), a methylxanthine derivative, is a well known inhibitor of pro-inflammatory cytokine production.14 The anti-inflammatory effects of PTX are mainly due to its action on TNF-α production as well as on cytokine-driven recruitment and transvascular migration of leukocytes.15 Interestingly, in an in vivo model of chronic liver fibrogenesis PTX have been reported to inhibit TNF-α-stimulated biosynthetic activities of fibroblasts and to increase collagenase activity16 and to have antifibrotic effects acting through down-regulation of profibrogenic cytokines and procollagen I expression.17 Since all these actions may be beneficial in the therapeutic management of NASH studies on the effects of PTX in this condition are emerging. In fact, a recent experimental study shows that PTX decreases serum ALT levels and hepatic inflammation in a dietary model of steatohepatitis, likely via increasing glutathione levels or reducing TNF-α expression.18 Moreover, two recent small-size clinical pilot studies suggest that PTX may be beneficial in patients with NASH.19,20 However, no data on the effects of PTX on liver fibrosis development in NASH is available. Thus, the aim of the present study was to investigate whether PTX can influence the early phase of fibrogenesis in an animal model of NASH.
MethodsAnimals and experimental protocol: Male Sprague-Dawley rats (170-190 g body weight) were housed in transparent polycarbonate cages, with wood chip bedding at a 12 h light/darkness cycle, a temperature of 21° C, and a relative humidity of 50% throughout the accommodation (at least 1 week) period and permitted ad libitum consumption of water. Choline-sufficient (CSD) or deficient (CDD) diets were obtained from Dyets Inc (Bethelem, Pennsylvania). NASH was induced as described21,22 by administration of a CDD during eight weeks. Animal experiments were approved by the Local Ethics Review Committee on Animal Experiments according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
PTX, sodium pentobarbital, 3-alpha-hydro xy steroid dehydrogenase and others organic chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All inorganic chemicals and organic solvents were obtained from E. Merck (Darmstadt, Germany).
Animals were randomized in three experimental groups: CSD group (control) fed a CSD, CDD group fed a CDD and CDD+PTX group fed a CDD and simultaneously receiving PTX (9 mg/kg body weight) in drinking water. All animals received diets for 8 weeks. After treatment, the rats were euthanized with a single dose of sodium pentobarbital Arterial blood samples were taken and livers were removed, snap-frozen in liquid nitrogen and stored at-70° C until analyzed.
Analytical procedures: Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using a Merck Diagnostica Kit (Darmstadt, Germany). Serum triglycerides, glucose and cholesterol were measured using a kit from Human Gesselheit (Wiesbaden, Germany). Total hepatic glutathione content was determined as described by Anderson et al.23
Histology and immunohistochemical examination: Sections 5 mm thick from the right lobe of all rats liver were routinely fixed in 10% formalin and embedded in paraffin. Then 4-mm-thick sections were stained with hematoxylin/eosin and 0.1% picrosirius red solution. For the detection of activated hepatic stellate cells, α-SMA was immunohistochemically assessed by the avidin-biotin-peroxidase complex method as previously described using an α-SMA monoclonal antibody.24 A blinded investigator evaluated the slides and assigned a score for steatosis, inflammation and fibrosis as described.18 In brief, score were given as it follows: steatosis: grade 0, none present; grade 1, steatosis of < 25% of parenchyma; grade 2, steatosis of 26–50% of parenchyma; grade 3, steatosis 51–75% of parenchyma; grade 4, steatosis > 76% of parenchyma. For inflammation; grade 0, no inflammatory foci; grade 1, < 5 inflammatory foci per high power field (hpf); grade 2, > 5 inflammatory foci/hpf. For inflammation: grade 0, no fibrosis, grade 1, pericellular fibrosis, grade 2, bridging fibrosis, grade 3, presence of nodules.
RNA isolation and quantitative reverse-transcription polymerase chain reaction (RT-PCR) assay: Total RNA was prepared from frozen liver tissues using SV total RNA isolation kit (Promega, Madison, WI, USA). The quantity and purity of RNA was verified by measuring absorbance at 260 and 280 nm. Further, the integrity of RNA was confirmed by electrophoresis on a formaldehyde-denaturing agarose gel. For RT-PCR assays, total RNA (5μg) was reverse-transcribed using SuperscriptTM First-Strand Synthesis System (Invitrogen, California, USA). Specific oligo-dT12-18 primers for alpha-1-procollagen and Transforming Growth Factor-beta (TGF-β) genes were used, according to Honda et al.25 18S primers (Ambion Company, Austin, TX) were used to quantitate transcript abundance. PCR was performed in the presence of alpha-32P-dCTP. PCR products were resolved on a 2% agarose gel and transferred to a nylon filter. Radiolabelled bands were quantified using Molecular Imager System GS-525 (Bio-Rad). Results were normalized to the signal generated from the radiolabelled 18S PCR product to correct for loading differences.
Statistics: All results are expressed as mean ± SE. A two-tailed non-paired Student’s t-test was used to compare differences between groups. Values were considered significantly different when the P value was equal to or less than 0.05.
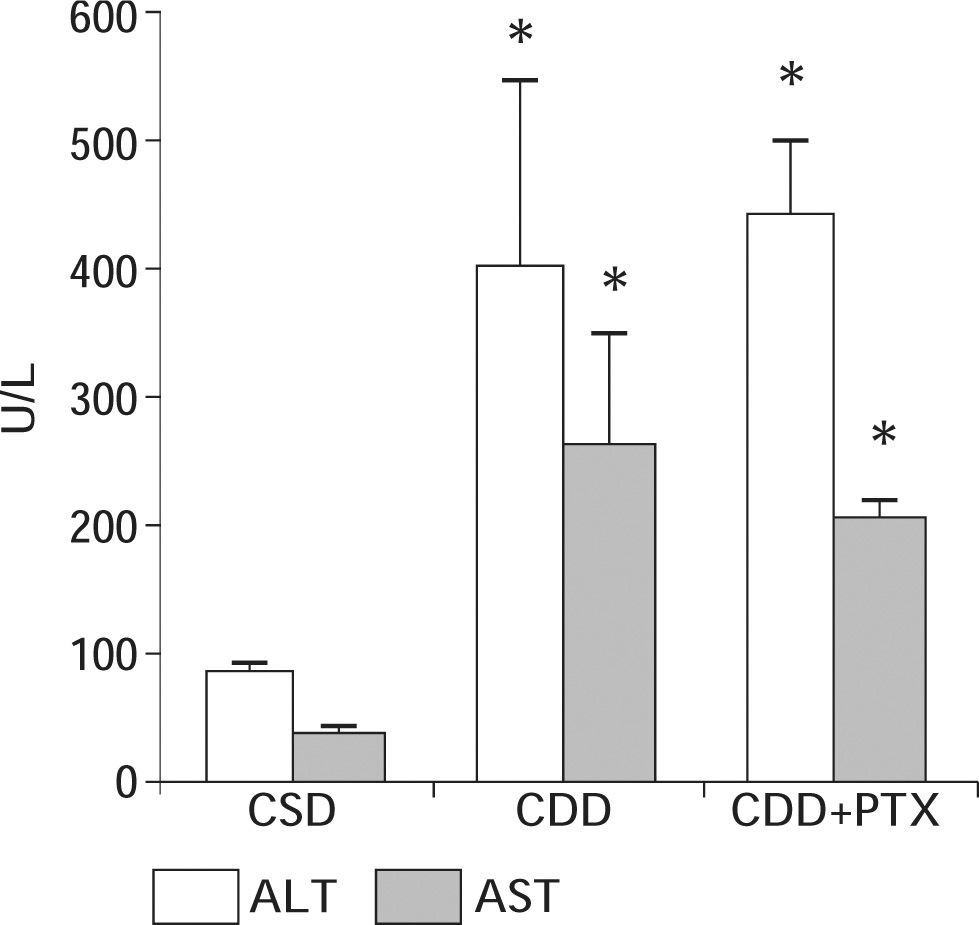
ResultsBody and liver weight, serum parameters and hepatic glutathione content: After eight weeks of diet administration all animals exhibited an increase in their body weight. However, animals on CDD and CDD+PTX gained significantly less weight than controls at the end of the experiments. The initial and final body weights of the experimental groups are shown in table I. After eight weeks of diet administration, the final body weight in groups CDD and CDD+PTX was significantly lower than that observed in control animals [p < 0.05] with no differences between CDD and CDD+PTX). Liver weights at the end of the experiment were significantly increased in CDD and CDD+PTX groups compared to controls (Table I). Values from the CDD and CDD+PTX groups were significantly greater than those seen in the control group [p < 0.05] with no differences between CDD and CDD+PTX. Dietary steatohepatitis induced by administration of the CDD diet was associated to an increase in serum ALT and AST levels (Figure 1). Observed values in experimental groups (CDD and CDD+PTX) were higher compared with those of the control group (p < 0.05). However, there were no differences between the animals treated with PTX and those not receiving the drug (Figure 1).
Body and liver weight, serum parameters and hepatic glutathione content in experimental groups.
| CSD (n = 5) | CDD (n = 5) | CDD+PTX (n = 5) | |
|---|---|---|---|
| Initial/Final Body Weight (g) | 201 ± 12/376 ± 13 | 200 ± 13/302 ± 13.5* | 200 ± 16/330 ± 12.8* |
| Final Liver Weight (g) | 10 ± 0.19 | 15.3 ± 1.0* | 18 ± 0.91* |
| Body/Liver weight ratio | 37.4 ± 1.1 | 19.8 ± 0.8* | 18.4 ± 0.7* |
| Glucose (mg/dL) | 108.5 ± 6.6 | 116.2 ± 5.9 | 105.7 ± 5.7 |
| Cholesterol (mg/dL) | 76 ± 3.7 | 48.3 ± 5.9* | 47.4 ± 9.5* |
| Bile Salts (uM) | 6.6 ± 3.6 | 31.2 ± 10.5* | 27.2 ± 2.6* |
| Triglycerides (mg/dL) | 74 ± 13.3 | 36.8 ± 5.4* | 22.1 ± 2.2* |
| Liver Glutathione (nmol/mg liver) | 4.5 + 0.5 | 2.9 + 0.3* | 2.5 + 0.18* |
Data represent means + SEM
Serum ALT and AST values in Sprague-Dawley rats after eight weeks of feeding with a choline deficient diet (CDD) with or without penpentoxifylline (PTX) supplementation. Control animals received a choline-supplemented diet (CSD). There was a significant increase in serum ALT and AST values in experimental groups with no effect of PTX. Data represent the mean ± SEM. *P < 0.01 compared to the CSD group.
Other parameters are shown in Table 1. Serum lipids levels (triglyceride and cholesterol) were significantly lower in experimental groups compared to control group with no differences found when comparing experimental groups. Plasma glucose showed no statistically significant differences between groups while serum bile salts were significantly higher in experimental groups compared to control group. Finally, administration of a CDD diet was associated to a significant reduction in hepatic glutathione content that was not prevented by PTX (Table I).
Histological findings: histological slides were evaluated by a blinded investigator and analyzed using a semiquantitative score for steatosis, inflammation and fibrosis. Both experimental groups (CDD and CDD+PTX) exhibited panlobulillar hepatic steatosis (Figure 2) with no apparent effect of PTX on this variable (steatosis score: 3.6 ± 0.1 vs 3.4 ± 0.1 in CDD and CDD+PTX groups respectively, NS). In addition, and in spite the significant elevation in serum ALT and AST levels, histological examination showed little inflammation in the experimental groups with no influence of PTX on this finding. Finally, histological evidence of mild liver fibrosis was observed in the form of fine porto-central and porto-portal bridges in both CDD and CDD+PTX groups (fibrosis 1.2 ± 0.1 vs. 1.2 ± 0.3 respectively, Figure 2). α-SMA-positive cells, which indicate activated HSC, were found only in experimental groups, with no significant difference between them (data not shown).
Hepatic histology after eight weeks of treatment with control or choline deficient diet (CDD) with or without pentoxyphilline (PTX) supplementation. A: red sirius stain of a control liver. B: hematoxyllin and eosin stain of representative CDD fed rat showing panlobulillar steatosis. C: red sirius stain of a liver section from a CDD fed rat. D: red sirius stain of a liver section from a CDD fed rat receiving PTX supplementation. Fine porto-central and porto-portal bridges in both CDD and CDD+PTX groups Representative livers are shown. (X200).
Transforming growth factor beta (TGb-1) and Procollagen-I mRNA levels: A significant increase in mRNA levels of TGF-β1 was seen after CDD feeding in experimental group that was not modified by PTX administration (Figure 3). mRNA levels of Procollagen I increased only in the CDD+PTX group.
Hepatic TGF-β and Procollagen I (Procol I) gene expression after eight weeks of treatment with a choline deficient diet (CDD) with or without pentoxyphilline (PTX) supplementation. Control animals received a choline-supplemented diet (CSD). There was a significant increase in liver TGF-β mRNA levels in rats treated with both CDD and CDD+PTX, compared to control animals indicating ongoing fibrogenesis. No significant differences in TGF-β mRNA expression occurred between the CDD and CDD+PTX groups. mRNA levels of Procol I increased significantly only in the CDD+PTX group compared to the control animals. Data represented as mean ± SEM. *P < 0.05 compared to controls.
The CDD model of NASH is associated to a marked liver injury and fibrosis development after eight to twelve weeks of diet administration.21 The aim of the present study was to determine if co-administration of PTX to rats fed a CDD during eight weeks was able to attenuate CDD induced liver damage. Our results showed that PTX failed to improve both steatohepatitis and liver fibrosis in this dietary model of NASH. In fact, no differences were observed in the experimental groups (CDD and CDD+PTX) compared to the control group with regard to weight loss, hepatomegaly, serum levels of aminotransferases, hepatic glutathione content, histological changes and markers of early fibrogenesis (i.e. procollagen I and TGF-β1 overexpression).
In spite of ample in vitro data on its antifibrotic ef-fects,26 PTX has been assayed in several in vivo models of liver fibrosis with conflicting results.17,27 Among other actors that likely influence the response to PTX treatment and may explain controversial results are the pathophysiological mechanism of the fibrosis model, the reactivity of the receptors to the agents, the presence of interfering cytokines and growth factors and bioavailability of the drug in sufficient concentrations in target tissues.27 Only one report assessing the effects of PTX in NASH has been published.18 In this report, the authors found, in contrast with our results, that PTX attenuated methionine-choline deficient diet-induced steatohepatitis. Although, the latter experimental model is similar to that used in the present study.28,29 a variety of factors, including species and/or sex of the animals, length of treatment [two weeks in the study by Koope et al.18 vs 8 weeks in our protocol] may explain the apparently conflicting results. It should be noted that in the study of Koope et al. beneficial effects of PTX were mainly observed in female mice indicating a possible gender specific effect. In the present study male rats were used with no observed benefit of PTX. Thus, the possibility that gender influences the efficacy of PTX therapy must be further evaluated.
In humans, two recent small pilot clinical trials have shown that aminotransferase levels among patients with NASH improve with administration of PTX.19,20 Larger studies are ongoing to define if PTX could be beneficial for patients with NASH. The results of the present study, suggest that although an initial attenuation of liver damage could eventually be achieved, PTX may not be able to influence liver fibrosis development in this increasingly important liver disease.
Acknowledgements: This work was supported by grants from the Fondo Nacional de Ciencia y Tecnología (FONDECYT #1050780 to MA).