Background. The rising incidence of non alcoholic fatty liver disease (NAFLD) mirrors the epidemics of obesity and metabolic syndrome. Primary care practitioners (PCPs) are central to management of patients with NAFLD, but data on knowledge and attitudes of PCPs towards NAFLD are lacking.
Material and methods. We conducted a statewide, stratified survey of 250 PCPs to examine knowledge, practices and attitudes regarding NAFLD and the barriers to providing care for this condition.
Results. NAFLD was perceived as an important health problem by 83% of PCPs. Eighty five percent of PCPs underestimated the population prevalence of NAFLD. Although the association of NAFLD with metabolic syndrome was identified by 91% of PCPs, only 46% screened diabetic obese patients for NAFLD. Only 27% of PCPs referred NAFLD patients to a hepatologist for evaluation. PCPs who reported seeing more than 5 NAFLD patients annually, referred to hepatology less frequently (P = 0.01). The majority of PCPs (58%) recommended weight loss and a calorie restriction. Only 8% of PCPs would recommend Vitamin E. The major perceived barrier in managing NAFLD was lack of confidence in understanding of the disease (58% of PCPs).
Discussion. An overwhelming majority of PCPs perceived NAFLD as an important health issue in their practice. However, screening rates for NAFLD among obese diabetics were low. A major barrier to managing these patients was self-reported lack of knowledge about NAFLD. Development of guidelines should emphasize strategies for screening vulnerable populations (obese, diabetics), evidence based management and barriers to providing care.
Obesity related diseases including non-alcoholic fatty liver disease (NAFLD) are increasing globally by epidemic proportions.1,2 In the last decade the prevalence of obesity has increased sharply in the US and in Wisconsin where the prevalence of obesity in adults was 26%.3 The resulting increase in diabetes, cardiovascular disease, cancer and other obesity related illnesses will have profound effects on health as well as health care related economy. NAFLD is closely related to metabolic syndrome, best characterized by diabetes and obesity, mirroring the increasing incidence of these conditio-ns.4,5 Over the next two decades NAFLD is projected to become the single most common indication for liver transplantation in the US.6,7 Treatment of the associated conditions is expected to have an impact on overall mortality, as NAFLD has been associated with increased cardiovascular and liver related mortality.8–12 Indeed, mortality attributed to cardiovascular issues among patients with NAFLD is higher than would be predicted based on the presence of metabolic syndrome alone.13–15
In a study of primary care patients evaluated for elevated liver enzymes, 26.4% were diagnosed with NAFLD and 7.6% had advanced fibrosis.16 As primary care offices are seeing an increasing burden of associated conditions (diabetes, hypertension, hyperlipidemia and obesity), this venue presents the best opportunity for identifying and evaluating a broad cohort of susceptible patients. Without adequate identification of individuals at risk, there is a significant loss of opportunity for early intervention. Other less common associations include celiac disease, which has been found in 3.% of NAFLD patients as the only manifestation of the disease.17 Identification requires knowledge about associated conditions, screening strategies, and a time investment to perform an evaluation. In addition, knowledge about subsequent management strategies including self-management and appropriate referral is key to reducing further morbidity and excess mortality.
In the literature, there is scant information about primary care providers’ awareness regarding NAFLD diagnosis and management.18
Given the growing significance of the problem, the important role of primary care and the scarcity of data on attitudes and knowledge regarding NA-FLD among PCPs, we conducted a survey among a representative sample of PCPs throughout Wisconsin to define their knowledge, awareness of management strategies, attitudes and perceptions regarding NAFLD, and the barriers to providing care for patients diagnosed with this damaging and prevalent condition.
Material and MethodsStudy sampleA master list of primary care physicians (Internal Medicine and Family Medicine) was obtained from the Wisconsin Department of Regulation and Licensing and used to select a random sample of 250 physicians from the entire state of Wisconsin. The sample was stratified by county and ZIP codes with the number of participants weighted by the population density of each county.
Survey instrument and data collectionWe developed a 20-question survey to assess providers practice, knowledge and attitudes regarding NAFLD (survey available upon request). Practitioners were asked if they had seen and diagnosed NA-FLD in the past year, if they screened patients with obesity and diabetes for NAFLD and if they referred patients with NAFLD to liver specialists. In addition, they were asked about NAFLD knowledge including prevalence in the general and obese population and diabetics, the association of NAFLD with metabolic syndrome and risk for progression. They were also asked about associated conditions such as sleep apnea and medications that can cause a fatty liver (e.g., methotrexate and amiodarone). Questions regarding NAFLD management included weight loss, appropriate caloric reduction, bariatric surgery, and Vitamin E. PCPs attitudes and perceived barriers in their practice regarding evaluation and management of NAFLD were examined. In addition, information on demographic characteristics and practice patterns of PCPs (place of practice, number of years of practice/recertification) was collected.
Survey responses were categorized by multiple-choice format with a single best answer elicited for some questions and more than one choice possible for others (e.g., NAFLD management strategies, NAFLD associated conditions, barriers to practice regarding NAFLD). There was also an opportunity to write in any barriers not pre-specified. Imposed categories were given for questions about number of patients seen (e.g., 0, 1 to 5, 6 to 10, > 10) and proportion of patients screened or referred (e.g., all, none, < 50%, > 50% but not all) as well as years in practice or since recertification (< 5 years, 6–10 years, 11–20 years).
The survey was evaluated for construct and content validity by expert review and pilot-tested on a local group of primary care practitioners.
A cover letter defining the term NAFLD (including analogous terms fatty liver disease, NASH), introducing the investigators and describing the concept of the study were mailed to providers along with the survey. Screening strategies for fatty liver disease including ultrasound, MRI, or liver enzyme profiles were outlined in the cover letter to PCPs. A second mailing was sent three months later with a token incentive ($5) to increase response rates.
Data analysisSurvey responses were tabulated as frequencies and percentages. For discrete data cross tabulations, Chi square statistic and Fishers Exact test were used. IBM SPSS v20.0 was used for data analysis.
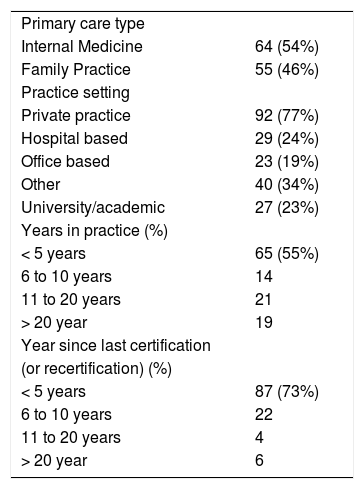
ResultsDemographicsOf the 250 surveys mailed, 210 were sent to active valid addresses; 119 providers returned a completed survey (57% response rate). Survey respondents were equally divided between Internists and Family Practitioners (Table 1). The majority were in non-academic practices (77%). Seventy three percent of the cohort had certified (newly certified or recertified) within 5 years.
Baseline characteristics of survey respondents (n = A 119).
| Primary care type | |
| Internal Medicine | 64 (54%) |
| Family Practice | 55 (46%) |
| Practice setting | |
| Private practice | 92 (77%) |
| Hospital based | 29 (24%) |
| Office based | 23 (19%) |
| Other | 40 (34%) |
| University/academic | 27 (23%) |
| Years in practice (%) | |
| < 5 years | 65 (55%) |
| 6 to 10 years | 14 |
| 11 to 20 years | 21 |
| > 20 year | 19 |
| Year since last certification | |
| (or recertification) (%) | |
| < 5 years | 87 (73%) |
| 6 to 10 years | 22 |
| 11 to 20 years | 4 |
| > 20 year | 6 |
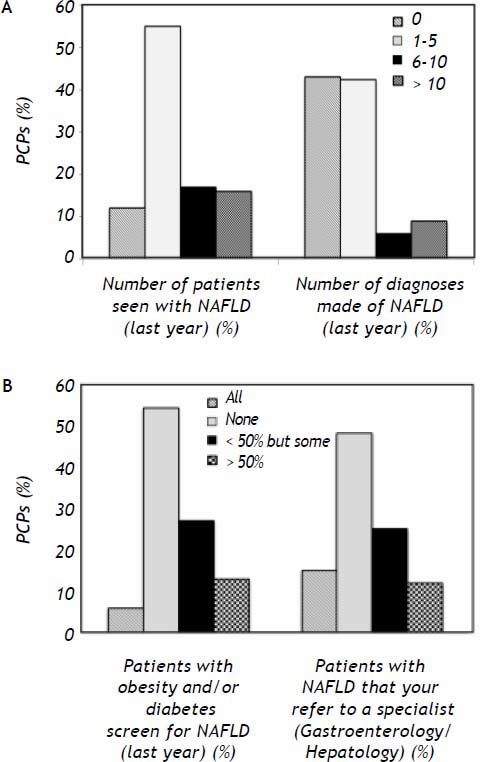
Of the respondents, 55% of PCPs reported seeing between 1 to 5 patients with NAFLD in the preceding year and 33% saw more than five such patients in their practice. New diagnoses of NAFLD were made by 57% of respondents, with 15% making more than 5 new diagnoses of NAFLD per year (Figure 1A).
Screening and referral practices- •
Screening. When asked if they screened patients with obesity and/or diabetes for NAFLD in their practice, 54% of respondents did not screen at all, 19% screened the majority or all these patients, and 27% screened fewer than half of these patients (Figure 1B).
- •
Referral. When asked if they referred patients with NAFLD to a specialist (gastroenterologist or hepatologist) for evaluation, 48% did not refer any patients, 15% referred all patients and 37% would refer some patients with NAFLD (Figure 1B).
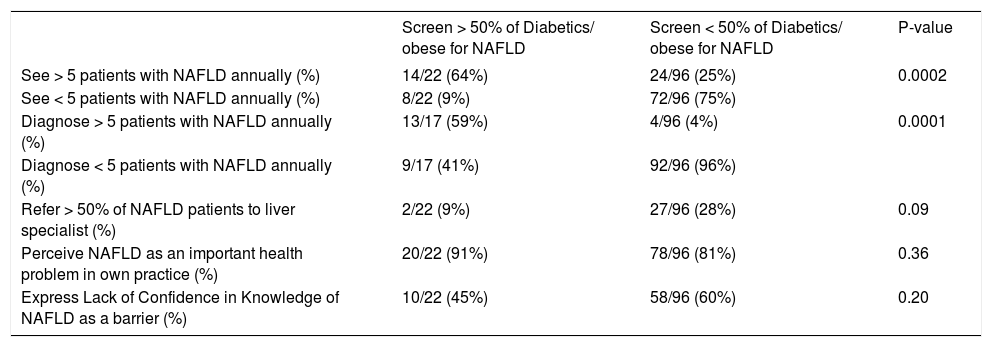
Compared to PCPs who screened less than 50%, those who screen more than 50% diabetic/obese patients for NAFLD were more likely to report seeing more than five NAFLD patients (P = 0.0002) as well as making new diagnoses of NAFLD in the preceding year (P = 0.0001) (Table 2).
Associations between screening behaviors (for NAFLD) in primary care practice.
| Screen > 50% of Diabetics/ obese for NAFLD | Screen < 50% of Diabetics/ obese for NAFLD | P-value | |
|---|---|---|---|
| See > 5 patients with NAFLD annually (%) | 14/22 (64%) | 24/96 (25%) | 0.0002 |
| See < 5 patients with NAFLD annually (%) | 8/22 (9%) | 72/96 (75%) | |
| Diagnose > 5 patients with NAFLD annually (%) | 13/17 (59%) | 4/96 (4%) | 0.0001 |
| Diagnose < 5 patients with NAFLD annually (%) | 9/17 (41%) | 92/96 (96%) | |
| Refer > 50% of NAFLD patients to liver specialist (%) | 2/22 (9%) | 27/96 (28%) | 0.09 |
| Perceive NAFLD as an important health problem in own practice (%) | 20/22 (91%) | 78/96 (81%) | 0.36 |
| Express Lack of Confidence in Knowledge of NAFLD as a barrier (%) | 10/22 (45%) | 58/96 (60%) | 0.20 |
PCPs who screen the majority (> 50%) of diabetics/obese patients for NAFLD referred them less often to a specialist (9%) in comparison to PCPs who screened less than half of diabetic/obese patients (28% of these PCPs referred most NAFLD patients to a liver specialist), P = 0.09 (Table 2).
PCPs who saw more than five NAFLD patients per year also referred to a specialist less frequently (11%) compared to those who saw fewer NAFLD patients (35%) (P = 0.01). PCPs who diagnosed NA-FLD more frequently also refer less frequently (6% as opposed to 32%), P = 0.05.
Knowledge of NAFLDThe majority of PCPs (84%) underestimated the prevalence of NAFLD in the general population and among the obese. Moreover, 49% of PCPs underestimated the prevalence of NAFLD among individuals with diabetes. Ninety-one percent (91%) of PCPs reported knowledge of the association between metabolic syndrome and NAFLD, and 60% reported knowledge of the metabolic syndrome as a risk factor for concomitant cirrhosis (Table 3).
Knowledge of survey respondents regarding NAFLD.
| N (%) | |
|---|---|
| Identify US prevalence of NAFLD | |
| Underestimate Prevalence of NAFLD in general population and obese. | 100 (84%) |
| Correctly Identify US prevalence of NAFLD in general and obese population (10–15% general, pop, 70–80% obese). | 19 (16%) |
| Identify Prevalence of NAFLD in patients with diabetes. | |
| Underestimate prevalence. | 58 (49%) |
| Correctly identify prevalence. | 61 (51%%) |
| Identify the association of metabolic syndromes with NAFLD. | |
| Yes | 108 (91%) |
| No | 11 (9%) |
| Identify metabolic syndrome as risk factor for cirrhosis in NAFLD. | |
| Yes | 72 (60%) |
| No | 47 (40%) |
| Identify conditions associated with NAFLD. | |
| Sleep Apnea | 83 (70%) |
| PCOS | 72 (61%) |
| Hypothyroidism | 50 (42%) |
| All 3 of the above | 25 (21%) |
| Identify medications associated. | |
| with fatty liver | 38 (32%) |
| Methotrexate | 49 (41%) |
| Amiodarone | 1 (1%) |
| All of the above | 45 (38%) |
| None of the above | |
Table 3 reports knowledge of conditions associated with NAFLD (sleep apnea, polycystic ovarian syndrome, hypothyroidism). Seventy percent of PCPs reported knowledge of an association between sleep apnea and NAFLD. While the majority of PCPs identified at least one of the medications associated with fatty liver that we inquired about (methotrexate,
amiodarone), 38% of PCPs did not identify any of the medications associated with fatty liver.
Of PCPs who screen the majority of diabetics for NAFLD, 41% would recognize the associated conditions (sleep apnea, Polycystic Ovarian Syndrome (PCOS), hypothyroidism) vs. 17% of PCPS who screen less than half of diabetics, P = 0.01. Also 27% of PCPs who screen the majority of diabetics for NAFLD also recognize the medications associated with fatty liver versus 11% of PCPs who screen less often, P = 0.05.
No relationship was observed between PCPs reported knowledge about NAFLD and the number of patients with NAFLD seen or diagnosed by PCPs. The number of years in practice, or from recertification, or type of practice did not correlate with NAFLD practice, knowledge and management questions.
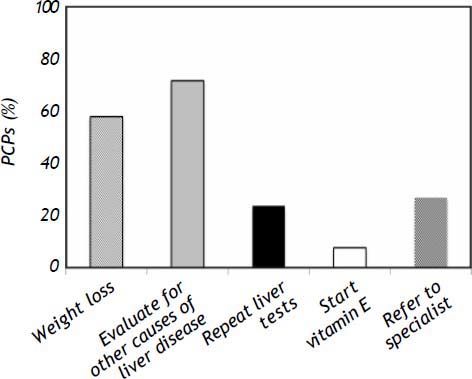
Management of NAFLDUsing a hypothetical case of a 39 year old obese male newly diagnosed with NAFLD based on ultra-sonography and elevated liver enzymes, we asked PCPs what their next step in management would be (allowing PCPs to choose more than one answer). Fifty eight percent of PCPs stated they would recommend weight loss as the first step in management of suspected NAFLD. A significant proportion (72%) would also evaluate for other causes of liver disease. Twenty seven percent would refer to specialist for further evaluation as an initial step. Only 8% would recommend Vitamin E as an initial step in management (Figure 2).
For dietary advice, a moderately calorie-restrictive diet (20% reduction in calories/day) was identified by 71% of practitioners as the best dietary strategy for NAFLD. Eighty percent of PCPs did not report knowledge of the benefits of vitamin E therapy on NAFLD liver histology and 41% indicated no therapy would be useful for histologic improvement in NAFLD. Gastric bypass was identified by 63% of PCPs as correlated with improvement in NAFLD in selected morbidly obese patients.
Of PCPs who see more than five NAFLD patients per year, 71% would recommend weight loss as their initial recommendation, significantly higher than those who see less than 5 NAFLD patients yearly (51% recommend weight loss), P = 0.04.
Of PCPs who diagnose NAFLD more frequently (> 5 cases per year), 35% recognized vitamin E as a therapy for NAFLD vs. 3% of PCPs who diagnose NAFLD less frequently, P = 0.0003.
Attitudes and perceptions of NAFLD
Among PCPs, NAFLD was perceived as an important public health problem in general (97% of PCPs) and in their own practices (83% of PCPs). The perception of NAFLD as an important problem in their practices was reported equally by PCPs who screened diabetics more frequently for NAFLD (91%) as well as those that screened less often (81%), P = 0.36. Screening rates for NAFLD were overall low. Of those PCPs who felt NAFLD was an important health problem in their practice, 21% (20/98) screened the majority of diabetic/ obese patients for NAFLD compared to 10% (2/20) of PCPs who did not think NAFLD was an important health problem in their practice (P = 0.36) (Table 2). In general, there was no correlation between perception of NAFLD as an important health problem and NAFLD knowledge, screening, and management questions (overwhelmingly PCPs considered NAFLD to be an important public health problem, independent of their practice and knowledge).
Barriers to evaluating and managing NAFLD
The major barrier in evaluating and treating patients with NAFLD (reported by 58% of PCPs) was lack of confidence in their knowledge about NAFLD and its management. Other barriers to managing patients with NAFLD included: time constraints (12% of PCPs); cost of evaluation and treatment (10%); outside the scope of PCP responsibility (7%); other issues taking precedence (2%); did not feel comfortable discussing obesity (2%); and perceived lack of compliance by patients (4%). Only 2% (2/119) felt NAFLD was not related to their patients’ health care maintenance.
The difference in self-reported lack of understanding of NAFLD as a barrier among PCPs who screened diabetics more frequently for NAFLD and those that screened less often was not statistically significant, P = 0.20 (Table 2). None of the other barriers correlated with screening rates for NAFLD (P > 0.20 for all).
There was no correlation between the referral rate to liver specialists and any of the reported barriers to evaluating and treating NAFLD in PCP practice (P > 0.2 for all).
DiscussionOf 47 million individuals in the US with the metabolic syndrome, 80% are estimated to have NA-FLD6,19 with the highest prevalence in the obese and diabetic populations.20–24 Identification of NAFLD in these populations has been endorsed by multi-disciplinary position papers both for adults25,26 as well as for children.27
Our study of PCPs demonstrated that an overwhelming majority reported NAFLD as an important public health problem, including in their own practices. The majority of respondents reported seeing NAFLD among patients in their practice in the recent past, and over 50% reported diagnosing patients with NAFLD themselves.
Although most providers underestimated the prevalence of NAFLD, particularly in the obese and diabetic populations, the majority was able to identify the metabolic syndrome as the strongest predictor of NAFLD and as a predictor of progression to cirrhosis. Identification of NAFLD-associated conditions, particularly sleep apnea, was frequently reported. However identification of some common medications that can cause fatty liver was relatively poor.
Despite this perception and knowledge regarding association with the metabolic syndrome, screening rates for NAFLD in diabetics and obese patients were low.
Kallman, et al. examined the attitudes of primary care physicians toward screening for viral hepatitis, and to a lesser extent, NAFLD.18 They demonstrated a lack of adherence to guidelines for screening of liver disease by PCPs. The study did not address provider’s ability to address long-term management issues, perceptions and knowledge of NAFLD.
Differences exist among recent published guidelines regarding actively screening for NAFLD in these populations. European and Chinese guidelines endorse screening for NAFLD utilizing imaging and liver enzymes in obese diabetics.28,29 Although recently published US guidelines (American Association for the Study of Liver Diseases) state that an argument can be made to screen for NAFLD in diabetes and obesity clinics, at this time they do not recommend screening for NALFD in primary care clinics due to uncertainties regarding diagnostic testing, treatment and long term outcomes.30
A majority of practitioners expressed lack of confidence in their knowledge regarding NAFLD as a major barrier in their ability to evaluate and manage these patients. Other important barriers to evaluation and management reported included lack of time, cost of evaluation and management, unclear PCP responsibility for screening as well as discomfort in discussing obesity with patients. Perceived lack of compliance with treatment was also mentioned as a barrier. Only in rare circumstances did PCPs state that NAFLD was not related to patients’ health care maintenance.
Those providers who reported screening and seeing more NAFLD patients referred to specialists less often. This is possibly related to confidence in their initial assessment. Given that the majority of PCPs would not refer to a specialist as the first step in management of their patients with NAFLD, a working knowledge regarding evaluation and management of NAFLD is essential. Referral to specialists is needed for assessment of prognosis (occasionally liver biopsy), and access to newer and experimental therapies and collaboration between specialist and primary care providers is endorsed for management of these patients.25,31
The proven therapies for NAFLD tackle the underlying metabolic syndrome and are often preven tive in nature. Weight loss though lifestyle intervention with a combination of exercise and appropriate caloric restriction is the mainstay of therapy.32 Vitamin E is a potential adjunct to weight loss in treatment of NAFLD. In a randomized controlled trial, vitamin E at a dose of 800 IU daily was found to cause significant improvement in the changes on liver biopsy associated with NAFLD.33 Use of insulin sensitizers such as the thiazolidinediones has shown conflicting data in larger trials and is not recommended for treatment of NAFLD.33–35 For selected patients with morbid obesity, bariatric surgery is a good option.36
The majority of these PCPs were able to correctly identify weight loss as an initial step in management as well as identify correctly the appropriate caloric restricted diet. Bariatric surgery was also recognized as useful in selected patients. However, only 8% of providers would recommend vitamin E as an initial step in management. PCPs may not agree with or know about recent trials showing efficacy of vitamin E for NAFLD in non- diabetics;33 concerns regarding prostate cancer and coronary artery disease with vitamin E supplementation may also impact the rate of vitamin E use overall although we did not explore this further.
Consensus guidelines can assist practitioners in evaluating and managing complex conditions such as NAFLD.37,38 Consensus guidelines have been developed by the Italian and Chinese Medical socie-ties28,29,39 and recently by the American Association for the Study of Liver Diseases (AASLD).39 These guidelines and positions papers endorse collaboration between specialists and liver specialists for management of NAFLD patients. However, even if guidelines exist, practitioners are often unaware of their existence40 and barriers to their use exist. Time constraints, cost, lack of self-efficacy about current treatments, lack of outcome expectancy and inertia of previous practice are common barriers affecting guideline use in general.40 In some studies on obesity, professionals have reported reluctance in discussing weight related topics and do not view obesity as a chronic disease.41 Practitioners also identify lack of qualification to support self-management and perceived lack of motivation and awareness among patients as barriers to implementation of practice guidelines for obesity management. The complexity of behavioral change required, as well lack of sup port and awareness at multiple levels are barriers in following guidelines for treatment. As noted in the results, respondents to our survey also reported some of these barriers.
Future DirectionsWe have identified important barriers to the diagnosis and management of individuals with NAFLD in the primary care setting. Future work should examine best ways to impart education and emphasize the role of PCPs in management of NAFLD.
AcknowledgmentsGuarantor of the article: Adnan Said, MD, MS takes responsibility for the integrity of the work as a whole, from inception to published article.
Specific Author ContributionsAuthor roles:
- •
Adnan Said was involved in study concept and design, data acquisition, analysis and interpretation and drafting of the manuscript.
- •
Veronika Gagovic was involved in data acquisition, drafting of manuscript.
- •
Kristen Malecki was involved in data analysis and interpretation and drafting of the manuscript.
- •
Marjory Givens was involved in data analysis and interpretation and drafting of the manuscript.
- •
Javier Nieto was involved in data analysis and interpretation and drafting of the manuscript.
None of the authors have any conflicts of interest to disclose.
All authors approved the final version of the manuscript and have no conflicts to disclose.