Introduction. Hyponatremia complicates cirrhosis and predicts short term mortality, including adverse outcomes before and after liver transplantation.
Material and methods. From April 1, 2008, through April 2, 2010, all adult candidates for primary liver transplantation with cirrhosis, listed in Region 11 with hyponatremia, were eligible for sodium (Na) exception.
Results. Patients with serum sodium (SNa) less than 130 mg/dL, measured two weeks apart and within 30 days of Model for End Stage Liver Disease (MELD) exception request, were given preapproved Na exception. MELD Na was calculated [MELD + 1.59 (135-SNa/30 days)]. MELD Na was capped at 22, and subject to standard adult recertification schedule. On data end of follow-up, December 28, 2010, 15,285 potential U.S. liver recipients met the inclusion criteria of true MELD between 6 and 22. In Region 11, 1,198 of total eligible liver recipients were listed. Sixty-two (5.2%) patients were eligible for Na exception (MELD Na); 823 patients (68.7%) were listed with standard MELD (SMELD); and 313 patients (26.1%) received HCC MELD exception. Ninety percent of MELD Na patients and 97% of HCC MELD patients were transplanted at end of follow up, compared to 49% of Region 11 standard MELD and 40% of U.S.A. standard MELD (USA MELD) patients (p < 0.001); with comparable dropout rates (6.5, 1.6, 6.9, 9% respectively; p = 0.2). MELD Na, HCC MELD, Region 11 SMELD, and USA MELD post-transplant six-month actual patient survivals were similar (92.9, 92.8, 92.2, and 93.9 %, respectively).
Conclusion. The Region 11 MELD Na exception prospective trial improved hyponatremic cirrhotic patient access to transplant equitably, and without compromising transplant efficacy.
In 2002 in the United States, the Model for EndStage Liver Disease (MELD) score became thestandard for determining liver allocation for transplantation.1 MELD was practical and quickly validated at a national level by its transparent and collegial development; and because it relied on three objective and reproducible, laboratory tests that were considered routine.2,3 Serum sodium (SNa) was not part of the original MELD calculation. Numerous high quality studies in patients with cirrhosis have demonstrated that hyponatremia can predict short-term mortality independently of other predictors, including MELD score.4-13 Furthermore, using United States’ data derived from all adult candidates for first-time liver transplant, registered with the Organ Procurement and Transplant Network (OPTN) in 2005 and 2006, the assignment of transplant priority using MELD score combined with serum sodium could have prevented death on the waiting list in 32 patients out of a total 477 patients (7%) whom died within 3 months of transplant waiting list registration.14 In this study, theRegion 11 (R11) Liver Program Directors developed a prospective MELD Na exception study, with United Network of Organ Sharing (UNOS) approval and Region 11 prospective oversight. The main objective was to determine whether a MELD Na exception would improve access to transplant for these highrisk hyponatremic and cirrhotic patients without unfairly disenfranchising other transplant eligible patient groups.
Material and MethodsFrom April 1, 2008 through April 2, 2010, patients listed for transplant in Region 11 with hyponatremia were eligible for a Sodium Exception. Patients with two serum sodium (Na) of 130 meq/L or less, at least two weeks but less than thirty days apart, were granted approval for the Sodium Exception. MELD Na was calculated as follows:4
[MELD + 1.59 (135-serum NA/30 days)]
MELD Na score was capped at 22 and ascites was not graded. Re-certification followed the UNOS Adult Liver Candidate Reassessment and Recertification Rules: A MELD Score ≤ 24 but > 18 requires recertification every month with laboratory values of the patient no older than seven days. Patients from all other regions continued to be listed per UNOS policy regardless of serum sodium values.
On December 28, 2010, data for this analysis was obtained from UNOS and analyzed by the unbiased statistician co authors (AM H and MKB). All patients had a minimum of six months of follow up from time of listing. Univariate comparisons were performed using х2 test of proportions or t-test, as appropriate. Kaplan-Meier post-transplant patient survival curves were constructed and compared with a log-rank test. For Table 1, 95% confidence intervals, were constructed from the student’s t distribution for continuous variables and from the binomial distribution for categorical variables. Four sample comparisons were evaluated using analysis of variance (ANOVA) for continuous variables and х2 test for contingency tables for categorical variables.
Demographic characteristics of each group.
| R11 Na excp. (n = 62) | R11 HCC excp R11 (n = 313) | R11 Std MELD (n = 823) | USA Std MELD (n = 13,212) | p-value (4-sample ANOVA) | |
|---|---|---|---|---|---|
| A | B | C | D | ||
| Age | 53.7 ± 8.6 | 54.8 ± 12.1 | 52.1 ± 11.7 | 51.9 ± 14.3 | 0.003 |
| (mean yrs) | (52.6, 54.8) | (54.1, 55.5) | (51.7, 52.5) | (51.8, 52.0) | (A & B > C & D) |
| A. American | 4 (6.5%%) | 34 (10.9%) | 104 (12.6%) | 1,097 (8.3%) | 0.0002 |
| (%) | (1.8, 15.7) | (7.6, 14.8) | (10.4, 15.1) | (7.8, 8.7) | C > D |
| Male | 38 (61.3%) | 234 (74.7%) | 541 (65.7%) | 8,535 (64.6%) | 0.002 |
| (%) | (48.0, 73.4) | (69.6, 79.5) | (62.4, 69.0) | (63.5, 65.4) | B > C & D |
| List Outcomes: | |||||
| Transplanted | 56 (90%) | 303 (97%) | 403 (49%) | 5,319 (40%) | < 0.0001 |
| (80.1, 96.4) | (94.2, 98.5) | (45.5, 52.4) | (39.4, 41.1) | A & B > C > D | |
| Died/Too sick | 4 (6.5%) | 5 (1.6%) | 57 (6.9%) | 1,195 (9.0%) | < 0.0001 |
| [1.8, 15.7] | [0.5, 3.7] | [5.3, 8.9] | [8.6, 9.5] | B < C & D | |
| Still listed | 1 (1.6%) | 3 (1.0%) | 311 (37.8%) | 5,606 (42.4%) | < 0.0001 |
| [<0.01, 8.7] | [0.1, 2.8] | [34.5, 41.2] | [41.6, 43.3] | A & B < C < D | |
| Time to Tx (Mean days) DD only | 111.7 ± 120 | 66.5 ± 97.8 | 121.1 ± 130.4 | 133.4 ± 139.6 | < 0.0001 |
| (96.3, 127.1) | (61.0, 72.0) | (116.5, 125.7) | (132.2, 134.6) | B < A, C, D A, C < D | |
| Na at time of Tx | 129.5 ± 4.5 | 137.3 ± 3.7 | 136.0 ± 4.4 | 136.6 ± 4.8 | < 0.0001 |
| (128.9, 130.1) | (136.8, 137.5) | (135.8, 136.2) | (136.56, 136.64) | A < B, C, D | |
| Patient survival | 92.90% | 92.80% | 92.20% | 93.90% | NS |
| (180 day) |
R11: region 11. Na: sodium. HCC: hepatocellular carcinoma. Std: standard. MELD: Model for End Stage Liver Disease. USA: United States of America. A. American-African American. Tx: transplant. DD: deceased donor. N/S: not significant.
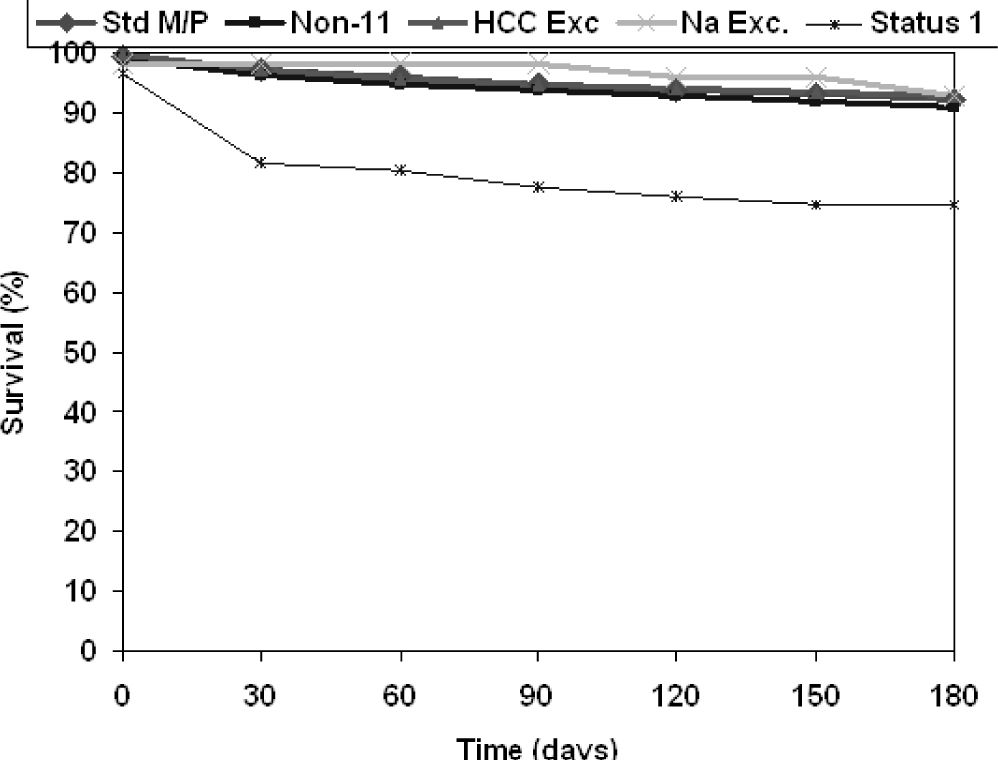
Between April 1, 2008 and April 2, 2010, a total of 22,677 patients were listed for liver transplant. Of these, 15,285 patients met the inclusion criteria of true MELD between 6 and 22. Demographic characteristics of the patients are shown in table 1. In Region 11, 1,198 patients were listed. Of those, sixty-two (5.2%) patients were eligible for Sodium Exception, (MELD Na), 313 (26.1%) patients for HCC exception (HCC MELD), and 823 (68.7%) were assigned standard MELD (SMELD). Over the same time period, 13,212 patients were included from the other regions (USA MELD). Age at listing, proportion of African-Americans, and proportion of males were not different between groups. The Region 11 exception groups were transplanted faster than both the SMELD and USA MELD groups: 90% of MELD Na and 97% of HCC MELD patients received a transplant by the end of the follow up period compared to 49% of SMELD and 40% of USA MELD (p < 0.0010), with comparable dropout rates (6.5, 1.6, 6.9, and 9%, respectively, p = 0.2). Mean wait times were 111 days for the MELD Na group, 66 days for the HCC MELD group, 121 days for the SMELD group and 133 days for the USA MELD group (p < 0.001). Six-month post-transplant patient survival is shown in figure 1, with Status 1 patient-survival shown for comparison. MELD Na, HCC MELD, SMELD, and USA MELD groups had comparable 6-month survival (92.9, 92.8, 92.2, and 93.9%, respectively), while 6-month survival in the Status 1 patients was only 74.6% (p = 0.03).
Post-transplant patient survival. Std M/P: standard MELD/patients (Pts) in region 11. Non 11-standard MELD/Pts in the USA. HCC Exc: hepatocellular carcinoma MELD exception/Pts in region 11. Na Exc: Sodium MELD exception/Pts in Region 11. Status 1: designation to acute liver failure Pts and hepatic artery thrombosis post liver transplant Pts given highest organ availability in Region 11. * Status 1 Pts survival at 6months: 74.6% (p = 0.03 < 4 other Pt groups).
Prior to 2002, HCC patients were sometimes unnecessarily prioritized, which disenfranchised non-HCC patients with equivalent mortality risk on the waiting list. Establishment of objective criteria for awarding MELD exception points was successful in the HCC population. There existed considerable evidence that an objective, reliable, and routinely available serum Na test could help better determine mortality risk in cirrhotic patients on the transplant list with equal MELD scores. Single center studies, including our own in a US veteran population, showed that a serum Na of < 135 meq/L was the single most important variable, in multivariable analysis, with significant predictive value for 180 day mortality in 12% of the cirrhotic population with MELD scores < 21.6,7 Although the majority of patients removed from the United Network for Organ Sharing/Organ Procurement and Transplantation Network waiting list for death or being too sick have high MELD scores, the addition of serum Na would benefit the most at risk for death waiting list population underserved by MELD.15
In 2005, and 2006, at the VCU Health Center, the waiting list mortality of 16% (22 patients) and 14% (16 patients) respectively, consisted of one half late referred high MELD liver failure patients and one half low MELD patients (MELD 14 to 21). We reasoned that patients we were caring for already with low MELD who could be more accurately risk stratified on the waiting list could be benefited with greater probability by affecting allocation policy compared to changing the complex variables involved in late referral patients not in our direct care.4-7
The desire to prevent inequity to the deserving listed 15 to 21 MELD population due to overcompensation to serve a few underserved low MELD patients resulted in the present study; a prospective MELD Na study exception protocol created over 1 year, 2007, of quarterly Region 11 Liver Program Director study development. The first model required a single serum Na of less than 135 meq/L and capped the MELD score at 26 with graded ascites. After three iterations, the present study protocol was accepted and implemented. This study had UNOS approval, automation of patient listing, data retrieval with quarterly safety oversight, and study initiation on April 1, 2008. The study design included two senior statisticians with transplant expertise who where tasked with performing quarterly analyses as agreed to by all Principle Investigators. The stop study date decision was to be determined by these two senior analysts (A.M.H. and M.K.B) in concert with the Principle Lead Investigator (R.A.F) once it was concluded that the three principle study aims could be answered conclusively by the study cohort:
- •
Would the volume of MELD Na exception patients approximate 7 to 8% predicted compared to non-Exception MELD ≤ 22, and other MELD exception patients?
- •
What wait list outcomes (transplantation; died/ inactivated; still waiting) could be anticipated in MELD Na exception patients and MELD equivalent non exception patients, due to the study design?
- •
What effect would MELD Na exception study have on post transplant patient groups survival?
The 11 UNOS regions vary not only in access to liver transplantation and end stage liver disease population event rates, but also in peer review liver allocation practice.16-18 An important issue addressed by this study is the number of patients who might be affected by nation-wide implementation of a Na MELD exception. Over the 2 years of prospective study in Region 11, of the 885 patients listed (excluding HCC exceptions), 62 patients (7%) were listed with the MELD Na exception. This 7% figure mirrors our original affected estimated population prediction and exactly that of a previous study that estimated that 7% of deaths on the wait list could have been avoided with the implementation of MELD exception for hyponatremia.14 This supports our hope that we have developed an effective MELD allocation modification that appropriately prioritizes those patients on the wait list underserved by a MELD score that underestimated this group of patients’ risk of death without harming their “less sick” equal MELD “neighbors”. Our original estimate of 7% MELD Na exception patients out of the total Region 11 MELD ≤ 22 listed patients to study, correctly influenced the study to not randomize MELD Na exception patients to receive the 22 points or to receive no Na calculated MELD points. This direct comparison study would have required more than 5 years of study. Instead, by giving all hyponatremic patients (Na ≤ 130) the MELD exception and demonstrating a drop in wait list mortality for the exception and the standard MELD group below the 14 to 16% wait list mortality in Region 11 centers, we could show indirect evidence that MELD Na was effective.
It is important to note that, as shown in table 1, the Region 11 MELD Na exception modification did not significantly, negatively impact the wait list mortality rate for the other groups of transplantation eligible patients. The mortality rate for the HCC MELD and SMELD groups in Region 11 were equivalent to national rates where no MELD Na was being made. The MELD Na group saw a 90% transplant rate, which is still less than that of the HCC MELD group (97%), but, as hoped, significantly higher than the rate seen in either the SMELD group (49%) or the USA MELD group (40%) (p < 0.001) (Table 1).
At the outset of the study there were concerns that the MELD Na exception would mean that hypo-natremic patients would be transplanted ahead of patients with HCC. Our study indicates that, not only were HCC patients still transplanted at the highest rate, but that HCC MELD patients were transplanted with a significantly shorter wait list time of 66 days as compared to 111 days for MELD Na pts, 121 days for SMELD patients, and 133 days for USA MELD patients (p < 0.001). The wait list mortality for standard Region 11 MELD patients (6.9%) was no different that the wait list mortality for Region 11 MELD Na patients (6.5%) or wait list mortality for USA standard MELD patients (9.0%). More importantly, of the 1,395 patients dying or removed from the wait list of USA standard MELD patients, 202 patients (16.9%) had Na ≤ 130 and a MELD score < 21 that could be salvaged by a National MELD Na policy with no increase risk of death to USA MELD patients who do not qualify for exception points.
To allay fears among some Program Directors that the serum Na would be manipulated to advantage some patients, our exception required that the serum Na be measured twice with at least two weeks separating the measurements and that both measurements demonstrated a serum Na < 130 meq/L. These requirements may be more conservative than necessary considering that there is an extensive body of literature that supports the idea that a serum Na < 135 meq/L predicts a more advance stage of cirrhosis, more significantly portal hypertension, more impaired body-water homeostasis, more profound liver dysfunction and a significant increased risk of death.4,7,19-23 While our requirements may have been overly conservative, they did result in a MELD Na group with very significantly depressed serum Na at the time of transplant as compared to the other groups (p < 0.001) that indeed were the true at risk underserved group by native MELD.
Previous literature suggests that hyponatremia pre-transplant is associated with significant morbidity, but not significant mortality, post transplant.24,25 Our prospective study did not study post transplant morbidity and found that the 6-month post transplant patient survival, at 92.9%, was not significantly different than any of the other groups (Table 1). We can conclude then, that the MELD Na exception did not reduce, transplant efficacy as measured by patient survival.
The Region 11 MELD Na exception as examined in this study was subsequently adopted, in 2011, as standard allocation practice by the Region 11 Liver Program Directors. Previous work in Region 11 studying ascites and hyponatremia in 211 Veterans with cirrhosis, suggested that patients with ascites on abdominal ultrasound may be incrementally disadvantaged in their access to transplantation when the laboratory MELD score is < 21.7 This finding was recently validated in a study of 18,124 US subjects listed for liver transplantation.26 Concerns regarding the objectivity of quantifying ascites were behind the decision to eliminate ascites from the present MELD Na exception. Future work may include investigations into the clinical relevance and impact of adjusting the MELD Na exception for the presence or absence of ascites.
This Region 11 cooperative, prospective MELD Na exception model took one year to plan and obtain a consensus study; two years to conduct; and is the first true multi-center test of the equity and efficacy of a MELD adjustment for hyponatremia at a Regional level, and may be viewed as a preliminary step to national implementation. Our results demonstrate that the MELD Na exception allows for a high rate of transplant for that patient group with excellent survival.
AbbreviationsRegion 11 UNOS organ allocation region made up of states of Kentucky, North Carolina, South Carolina, Tennessee, Virginia.
- •
MELD: Model of End-Stage Liver Disease score.
- •
MELD Na: Model for End-Stage Liver Disease + serum Na score.
- •
SNa: serum sodium.
- •
OLTX: adult orthotopic liver transplant.
- •
OPTN: Organ Procurement Transplant Network.