CD98 is a multifunctional glycoprotein that is involved in various biological processes such as amino acid transport, cell adhesion, diffusion, adhesion, and proliferation. The role of CD98 in liver disease has not thoroughly been examined and is limited reports in the literature. Among these reports, direct association for CD98 in nonalcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma (HCC) have been reported. Our lab has reported that targeting CD98 in high fat diet mice reduced steatosis and inflammation in NAFLD. Other reports associate CD98 in HCC due in part to the role of CD98 in activating integrin signaling. Herein, we present CD98 staining on liver biopsies from NAFLD, chronic active hepatitis, cirrhosis, and 3 stages of HCC to demonstrate the upregulation of CD98 expression throughout liver disease progression. In addition, we analyze current literature to elucidate roles and potential roles of CD98 with each stage of liver disease.
CD98 is a multifunctional glycoprotein protein consisting of a heavy chain and several light chains. CD98 has been implicated in a variety of biological functions such as cell fusion, differentiation, proliferation, adhesion, and migration [1,2]. This large distribution of function is due to the ability of CD98 light chain function for amino acid transport and heavy chain to act as a ‘molecular facilitator’ for activating integrin β1 and β3 signaling which suppression of CD98 led to impaired integrin β1 and β3 signaling [3,4]. The cytoplasmic domain and transmembrane domains of CD98hc are necessary for integrin interaction while the ectodomain is required for amino acid transport with the light chain [4,5]. Overexpression of CD98 alters the surface distribution of integrin β1 and demonstrates increased FAK and PI3K activity [6]. Interestingly, knockout of CD98 demonstrated that CD98 was not required for integrin expression, affinity, and adhesion; however, disruption caused defects in cell spreading and cell migration demonstrating that CD98 is necessary for efficient integrin β1 signaling [7,8]. CD98 was found to interact with CD147 to induce integrin β1 function to promote cyclosporine B-induced cell adhesion and p44/p42 MAPK activation following PI3K activation [9]. Although CD98 has been studied in a variety of biological processes, the role of CD98 in liver disease has not been extensively studied. Herein we will discuss the literature associated to the involvement and potential involvement of CD98 in liver disease.
2CD98 immunohistostainingA liver tissue array (LV1201) was purchased and the IHC staining service was requested from US Biomax inc (Rockville, MD, USA). IHC staining was performed with an automatic IHC stainer (Ventana) using CD98ab (Santa Cruz, Ref# sc-9160). Tissue samples were obtained as defined by the companies declaration of specimen collection.
3CD98 role in nonalcoholic liver diseaseFatty liver disease can be categorized into two groups: Alcoholic Fatty Liver Disease (AFLD) and NAFLD which are defined by steatosis as a result of overconsumption of alcohol and fatty foods [10]. NAFLD is typically an asymptomatic disease and is often reversible by lifestyle changes. NALFD has gained attention due to effects of the long-term pathogenesis of the disease in which unchecked inflammation and oxidative damage can lead to the progression to more severe liver diseases such as fibrosis, cirrhosis, and HCC [11].
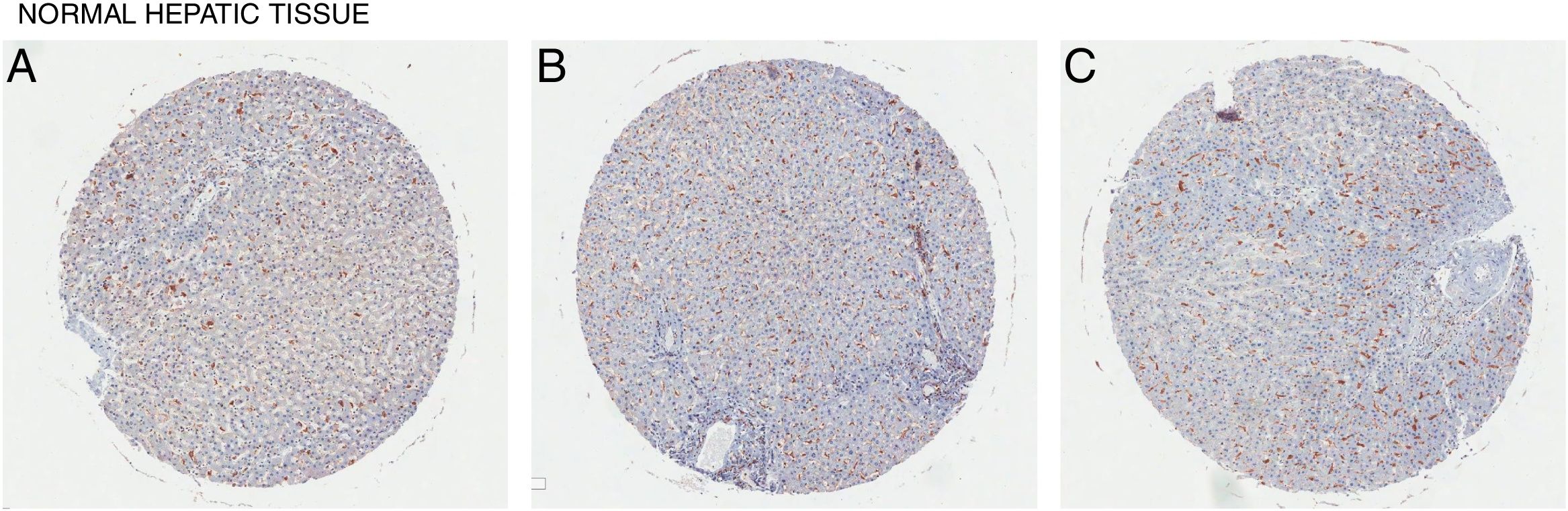
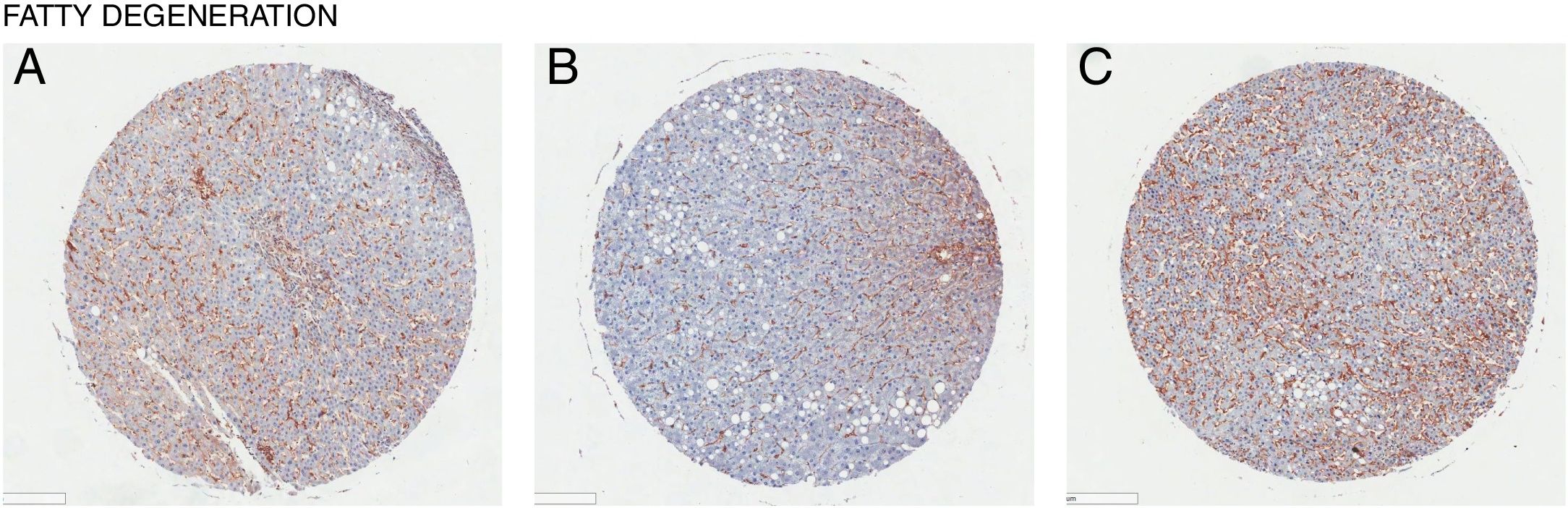
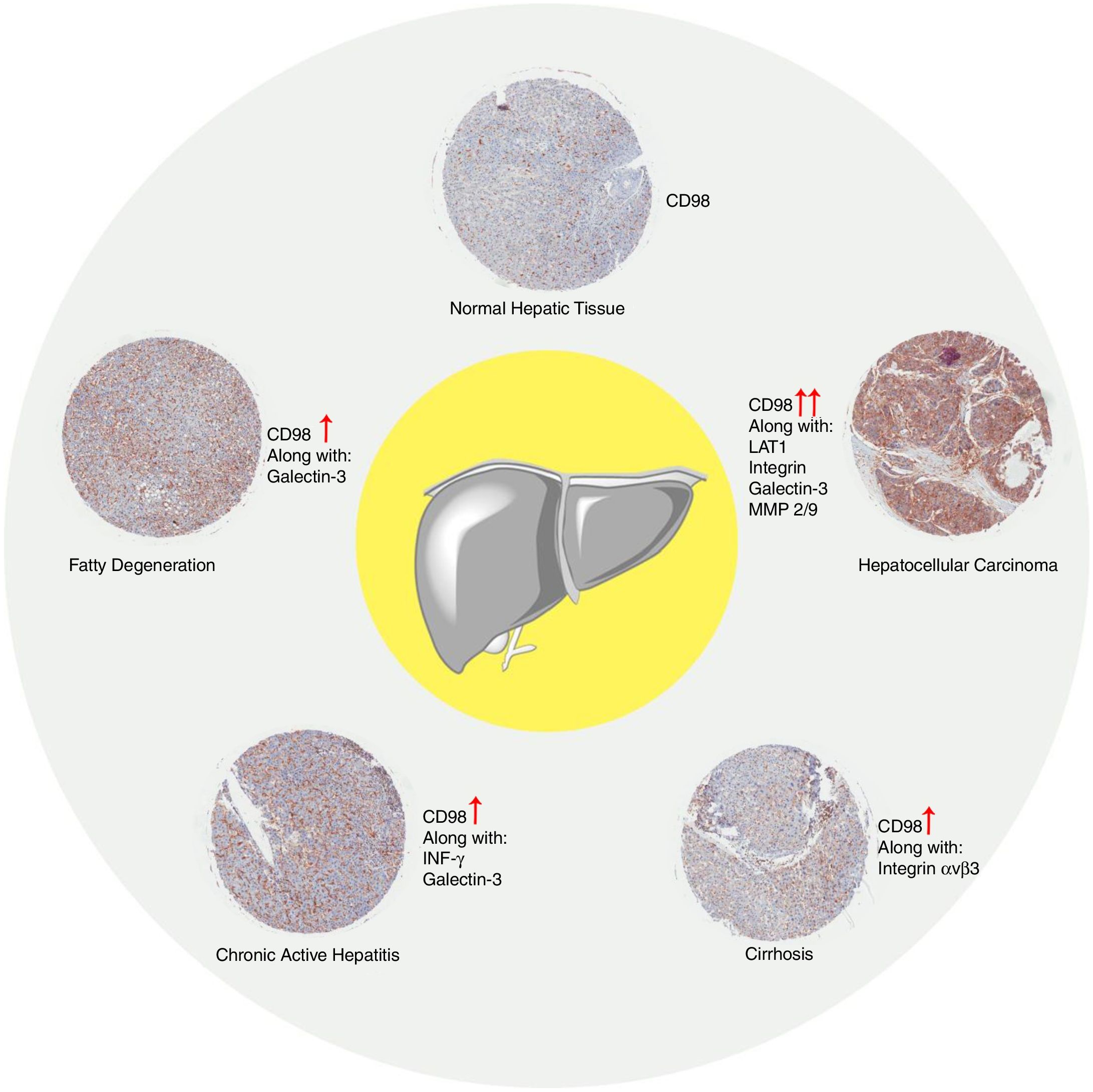
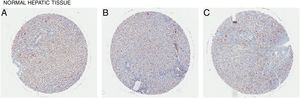
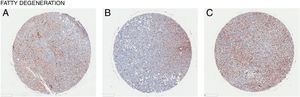
In all the figures presented in this review, liver samples have been stained by immunohistochemistry and CD98 appears brown on the pictures. Samples were then counterstained with hematoxylin. Patients with healthy livers (Fig. 1) exhibit basal levels of CD98 expression when compared to patients with fatty degeneration which exhibit upregulation of CD98 (Fig. 2). One method of CD98 involvement may stem from galectin-3. Galectin-3 has been shown to recognize surface glycoprotein receptors such as CD98 to induce CD98 activation [12]. Knockout experiments of galectin-3 have proposed 3 mechanisms as a consequence in the development of NAFLD: increased receptor for advanced glycation end products (RAGE) which promote inflammatory pathways, accumulation of advanced glycation end products (AGE), and increased expression of peroxisome proliferator-activated receptor γ (PPAR γ) [13]
Recently our lab has published a paper demonstrating that CD98 may function as a key actor/inducer in NAFLD [14]. Our study showed that downregulating CD98 expression via CD98 siRNA-loaded nanoparticles reduced inflammation and steatosis in the liver. In addition, fibrotic markers were seemingly attenuated. Taken together CD98 could represent a key therapeutic target for preventing the transition of NAFLD to the severe liver diseases. Our study was the first documented to show CD98 is implicated in NAFLD.
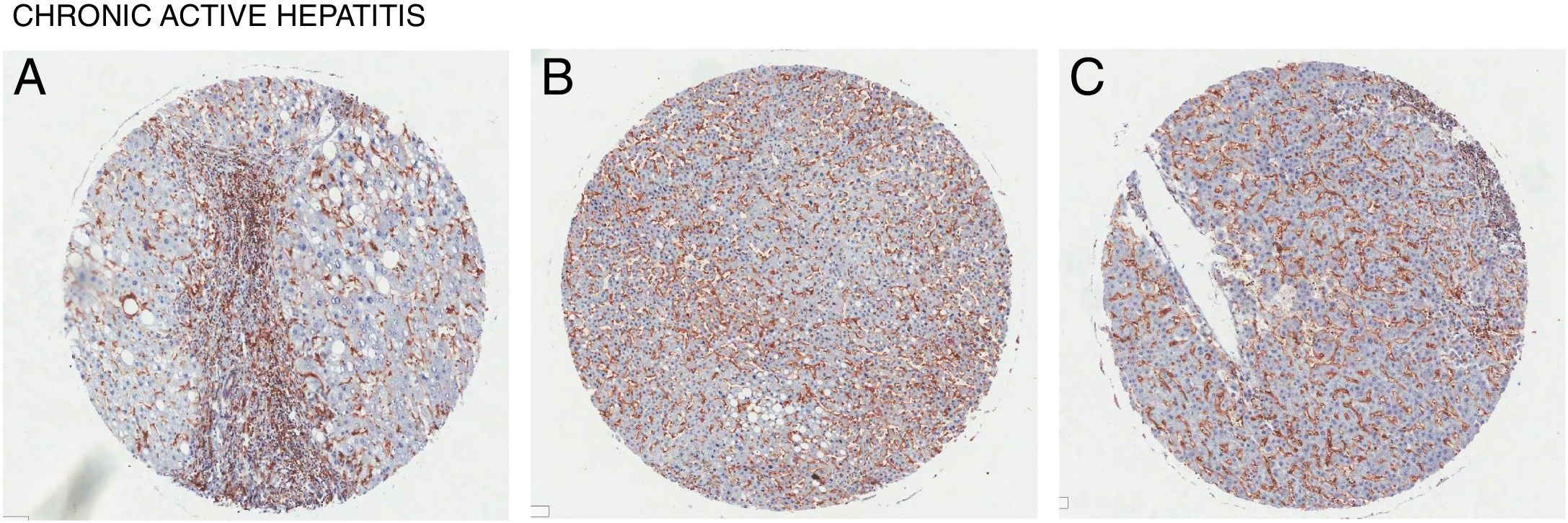
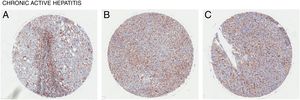
4CD98 role in nonalcoholic steatohepatitisNonalcoholic steatohepatitis (NASH) is characterized as a condition in which the liver has lobular inflammation in addition to steatosis and is grouped together with chronic active hepatitis [15]. NASH is generally asymptomatic and is frequently diagnosed in patients with metabolic syndrome. CD98 was found to be overexpressed in liver tissue of patients suffering from chronic active hepatitis (Fig. 3). However, direct involvement of CD98 has not been reported. One possible link for CD98 to the progression of NAFLD to NASH could be related with its role for T cell activation. The current 2 hit model for NAFLD pathogenesis links steatosis as the initial hit then subsequent inflammation and oxidation damage for the second hit where T cells play a role [16]. T cell activation during obesity seems to play a critical role in upregulating inflammatory cytokines, adiponectin, and lipogenic enzyme mRNA levels in the spleen and liver [17]. In this study, knockout and downregulation of CD28 seemingly improved liver steatosis and decreased mRNA levels of CD98. It has been reported that interferon γ (Inf-γ) is upregulated in NASH [18]. Inf-γ has been shown to induce the expression of epithelial CD98 which could possibly explain the observed expression levels [19]. Thus, CD98 upregulation in NAFLD could promote the progression to NASH via a similar mechanism.
Galectin-3 knockout mice prevented the development of NASH by down regulation of CD36 involved in fatty acid translocation and removal of ALE/AGE (advanced lipoxidation endproduct/advanced glycation endproduct) accumulation by the liver which is involved in inflammation [20]. Similarly, it was shown that using an inhibitor of galectin-3, GR-MD-02, prevented fat accumulation, inflammation, and fibrosis in mouse models of NASH due to decreased CD36 and ALE/AGE expression [21]. Activation of CD98 by galectin-3 can create a feedback loop for the PI3K activation which in turn produces IL4 that stimulates galectin-3 that will give rise to macrophage activation that is involved in disease pathology [22]. It is possible for the increased CD98 expression levels can stimulate galectin-3 for NASH progression.
5CD98 role in liver fibrosis and cirrhosisLiver fibrosis is a condition where there is an excessive accumulation of the extracellular matrix proteins which can lead to development of fibrous scar tissue. Fibrosis in the liver can progress in patients with either NAFLD or NASH albeit to a different degree [23]. CD98 expression levels could serve as the proinflammatory mediator for the persistent damage. Integrin αvβ1 was reported to bind TGFβ for latent TGFβ activation in tissue fibrosis [24]. Fibrotic progression in the liver could be linked to the activation of integrin αvβ1 via CD98. CD98's role in T cell activation in which the TGFβ activation could also play a profibrotic role [25]. Over proliferation of hepatic stellate cells has been linked to fibrogenesis [26]. It is possible that the CD98 levels may play a role in increasing the proliferation of the hepatic stellate cells via integrin activation. The only direct link is our report which showed that fibrotic markers on the liver were seemingly attenuated by downregulating CD98.
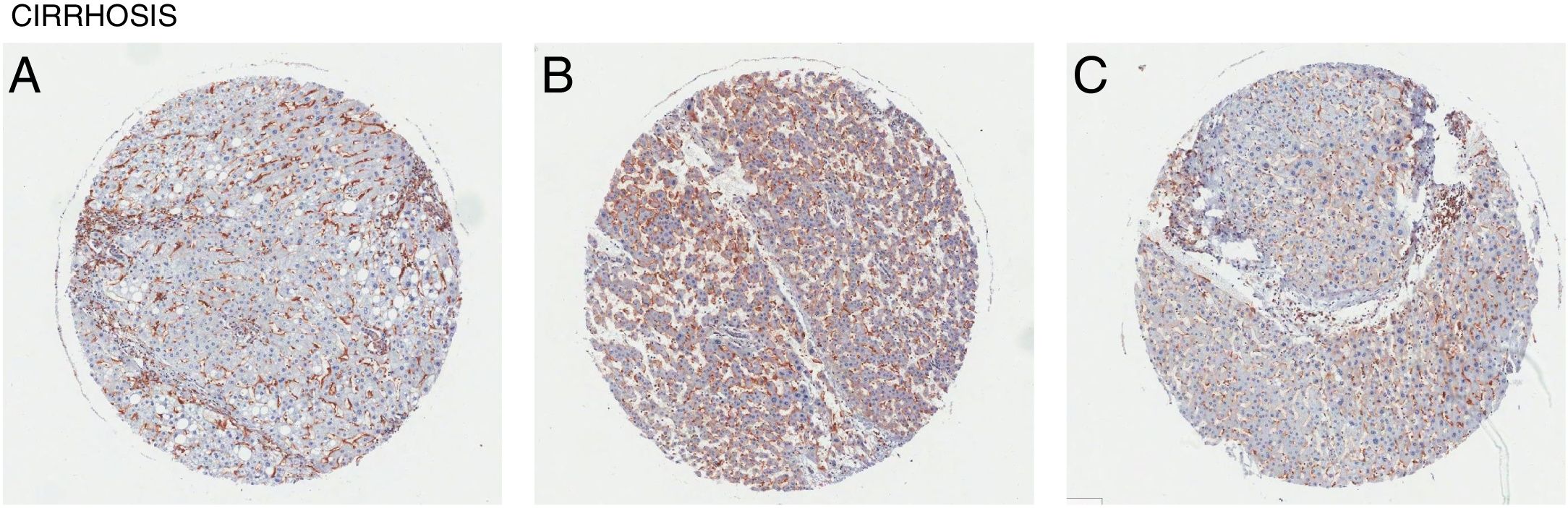
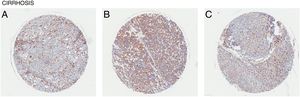
Liver cirrhosis is a condition where there is liver dysfunction due to the accumulation of liver damage often measured by excess scarring. Similarly, CD98 upregulation was observed in patient liver tissues with cirrhosis (Fig. 4). Like NASH and liver fibrosis, little is known about the direct involvement of CD98 in cirrhosis. Galectin-3 can be detected in patients with cirrhosis supporting that galectin-3 could play a role in the pathogenesis of NAFLD to more extreme liver diseases [27]. CD98 activation through galectin-3 may be a possible link.
6CD98 role in hepatocellular carcinomaCD98 heavy chain has been studied to be a key player in tumorigenesis due to its role in activating integrin signaling to promote tumor progression via angiogenesis, invasiveness, and proliferation in addition to promoting malignant transformation of cells [2,28]. In prolific endothelial cells, CD98 was found to be overexpressed and targeting CD98hc led to decreased EC-assisted tumor growth and angiogenesis [29]. Silencing of CD98hc also decreased malignant behavior and tumor growth in renal cell cancers via loss of downstream signaling due loss of FAK phosphorylation [30]. Crosslinking of CD98 was reported to increase the surface expression and clustering of integrin β1 as well as increase integrin-like signaling [31,32]. CD98 was further implicated in tumorigenesis via increased PI3K activity from downstream FAK phosphorylation from CD98-integrin signaling [6]. CD98hc-CD147 complexes present poor overall survivability in non-small lung cancer patients and knock down in cells lead to decreased Akt activity [33]. CD98hc silencing impaired tumorigenicity of HeLa cells via defects in galectin-3, β-catenin, and integrin signaling [34].
The CD98 light chains have also been implicated in tumor growth via activation of mTOR and providing essential amino acids tor the tumor [35]. Active LAT1 expression has been associated to transitional cell carcinoma progression in upper urinary tract and malignant pleural mesothelioma [36,37]. Overexpression of LAT1 was also observed in primary and metastatic sites of human neoplasms which correlated to the proliferation and angiogenesis [38]. LAT1 downregulation in tumor cells exhibited significant growth inhibition [39]. Considering the mechanisms of both the heavy chain and light chain, CD98 plays many roles in tumor behavior and development.
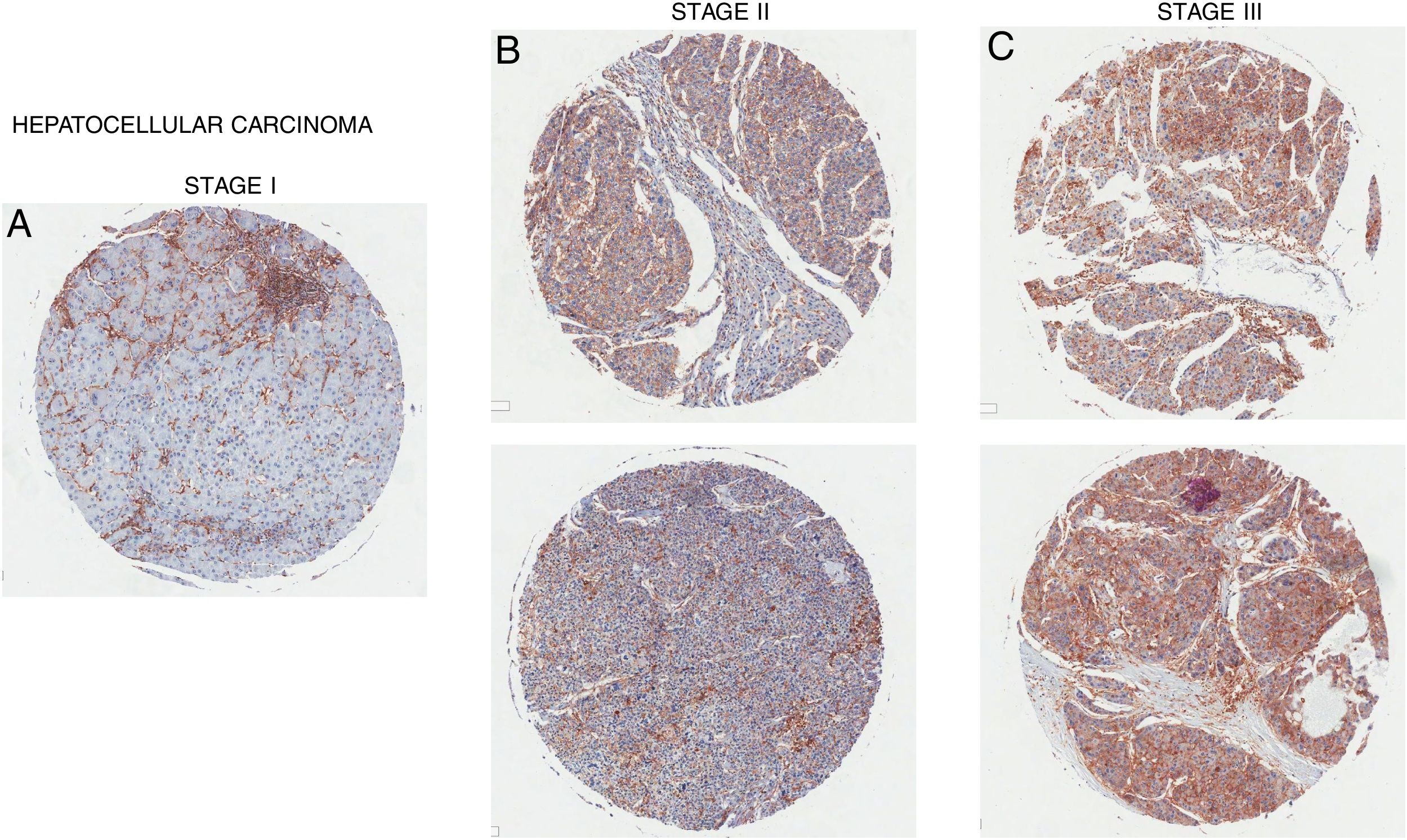
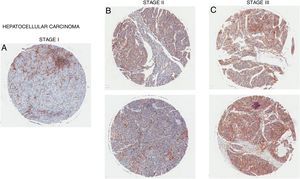
Overexpression of CD98 can be observed in patients with stage I, II, III hepatocellular carcinoma (Fig. 5). CD98 expression significantly increases with the severity of HCC. CD147 can redistribute CD98 to activate integrin signaling and through the upregulation of MMP2/9 for galectin-3 activation of CD98 in HCC [40]. Intracellular domain of CD98 truncations of CD98 exhibited inhibitory effect on integrin activation to prevent malignant development in HCC [41]. Another report linked the Lat1 to aiding tumor associated gene-1 with tumor progression in rat liver tumor cells [42]. LAT1 has been shown to promote tumor growth with CD98hc in rat liver tumors [43]. LAT1 was also reported to be a prognostic marker for patients with HCC and expression correlated with tumor size and stage [44,45]. In terms of metastasis, CD98 and LAT1 expression was found to be upregulated in metastatic sites on the liver in which LAT1 expression correlated to proliferation and angiogenesis [38]. Metastasis of the liver by colorectal cancer promotes the expression of CD98, integrin β1/β3, and Fak [46]. Thus, CD98 plays a role in the tumorigenesis of HCC and metastasis of the liver.
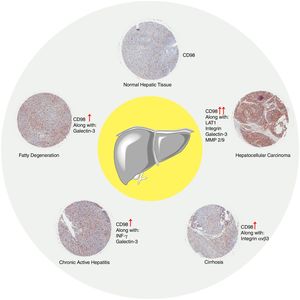
7ConclusionFrom what has been reported there is a clear gap in studies regarding CD98 involvement in liver disease in NAFLD, NASH, fibrosis, and cirrhosis. Our data presented that upregulation of CD98 occurred throughout the pathogenesis of NAFLD. Our report was the first to directly link CD98 to NAFLD. In addition, CD98 has been reported to play a role in HCC via integrin signaling. In our review, we identified potential areas for which CD98 could be involved with NASH, fibrosis, and cirrhosis which are summarized in Fig. 6. Further study will be needed to understand the underlying mechanism to explain the role of CD98 in NAFLD pathogenesis.
Author contributionsBrandon S.B. Canup.; draft preparation, Heliang Song; proofing and editing, Hamed Laroui; supervision and funding acquisition.
Ethical considerations and protocols used in tissue collectionThe tissue array (human samples of liver) has been purchased from US Biomax, Inc. (Rockville, MD). All tissues are collected under the highest ethical standards with the donor being informed completely and with their consent. US Biomax Inc. follows standard medical care and protection of the donors’ privacy. All human tissues have been collected under HIPPA approved protocols. All samples have been tested negative for HIV and Hepatitis B, and approved for commercial product development.
FundingThis work was supported by the Chemistry Department and the Center for Diagnostics and Therapeutics of Georgia State University as well as the National Institutes of Health of Diabetes and Digestive and Kidney Diseases through the grant K01-DK-097192 (to H.L.).
Conflicts of interestThe authors declare no conflict of interest.
We acknowledge the technical services provided by the Core Facility of Georgia State University. This work was supported by a grant from the NIH-NIDDK grant (K01-DK-097192 to HL).