Recurrent HCV infection after liver transplant (LT) has a negative impact on graft and patient survival. The aim of this study is to describe the efficacy and safety of sofosbuvir (SOF-based) regimens in the treatment of recurrent HCV after liver transplant (LT).
Materials and methodsThis retrospective study included 68 adults with recurrent HCV infection after LT, treated with different SOF-based regimens between March 2015 and December 2016. The choice of regimens, their duration and use of ribavirin (RBV) was made by the treating physician. The efficacy of antiviral treatment was assessed based on the sustained viral response obtained 12 weeks after the end of treatment (SVR12), according to an intention-to-treat analysis.
ResultsThe most frequent HCV genotypes were 1 and 3 (n=35, 51.4% and n=31, 45.6%, respectively). Only 22 patients were treatment naïve (32.3%) and 7 had cirrhosis (10.2%). SOF+daclatasvir (DCV) was the most commonly used regimen (n=63, 92.6%). Most patients used RBV (n=56, 82.3%) and were treated for 12 weeks (n=66, 97%). Overall SVR12 was 95.5% (65/68 patients). Three patients had virologic failure. Three patients had serious adverse events, however, no one discontinued treatment prematurely. RBV-related anaemia was the most frequent adverse event (n=34, 50%). Four patients had severe cellular graft rejection after HCV elimination, while immunosuppression remained stable.
ConclusionSOF-based therapy is highly effective and safe to treat HCV recurrence after LT. Cellular graft rejection following the successful treatment of HCV needs further investigation.
Chronic hepatitis C (HCV) infection is one of the main causes of liver disease worldwide [1]. The global prevalence of anti-HCV was estimated to be 1.6%, corresponding to 115 million people, of which 1.1% (85 million people) are viremic [2]. HCV related diseases, such as decompensated cirrhosis and hepatocellular carcinoma, are the main indications for liver transplant (LT) [3,4]. In Brazil, up to 70% of the adult liver transplants are due to HCV-related disorders [5]. Recurrent HCV infection is universal after LT and is often related to an accelerated course. Indeed, cirrhosis can occur in up to 30% of LT recipients within a 5-year period, jeopardizing the function of the graft and patient survival [6]. Treating recurrent HCV may ensure the survival of both the graft and patient [7–14].
Recently, the widespread use of new oral antiviral drugs, such as sofosbuvir (SOF), simeprevir (SMV) and daclatasvir (DCV) has led to high rates of cure in different populations, including LT recipients. Although the small number of patients included in most studies of HCV-recurrence after LT means definitive conclusions to be drawn, clinical trials consistently show SVR rates of over 90%. Moreover, the new oral drugs are safer than the previous interferon-based regimens [15–17] and the real-life studies performed so far provide encouraging results [18–21]. To the best of our knowledge, no study on therapy for post-liver transplant recurrent hepatitis C in Brazil has been published to date.
The aim of this study is to evaluate the efficacy and safety of SOF-based therapy for the treatment of recurrent HCV infection in a university hospital in Brazil.
2Methods2.1Study design and patientsThis retrospective study included real-life treatment of recurrent HCV infection after LT in a single centre in Brazil. All the transplants were performed using grafts from deceased donors. We included patients aged 18 years or over with any stage of liver fibrosis, regardless of previous treatment, who had received treatment for recurrent HCV after liver transplantation based on SOF in combination with NS5A inhibitor (DCV) or protease inhibitor (SMV), with or without addition of RBV, or SOF plus RBV only, between March 2015 and December 2016.
Immunosuppressive protocols consisted of a calcineurin inhibitor (cyclosporin or tacrolimus), an antimetabolite (mofetil mycophenolate) and prednisone. Prednisone doses were maintained exclusively during the first year after liver transplant or if it was required for the treatment of moderate or severe cellular graft rejection. Everolimus, a drug that inhibits mechanistic target of rapamycin (mTOR), was used for patients at high risk of post-transplant recurrence of hepatocellular carcinoma (HCC) or patients with renal injury induced by a calcineurin inhibitor.
The choice of the HCV antiviral regimen, its duration (12 or 24 weeks) and the use, reduction or suspension of RBV were determined in accordance with the recommendations of the Brazilian Ministry of Health Hepatitis C Therapeutic Guidelines. Daily doses of SOF (400mg), DCV (60mg) and SMV (150mg) were used. The dose of RBV was adjusted according to body weight (≥75kg: 1250mg/day or <75kg: 1000mg/day).
Electronic chart reviews were performed to obtain demographic data, HCV genotype, LT indication, current liver fibrosis, treatment regimens, laboratory tests, HCV viral load, immunosuppressive regimens and adverse events.
Fibrosis status was established by liver biopsy according to METAVIR scores and/or by transient elastography (F0<5.1kPa, F1≥5.1kPa, F2≥7.2kPa, F3≥9.5kPa and F4≥14.5kPa). Cirrhosis was defined by a liver biopsy, transient elastography or a combination of clinical findings and complications, laboratory test, imaging or endoscopy. The study conforms to the Ethical guidelines of the Declaration of Helsinki. The study was approved by the HCPA Ethics and Research Committee.
2.2Evaluation of efficacyThe primary endpoint was the proportion of patients who achieved undetectable levels of HCV RNA at week 12 after the end of therapy (SVR12). Viral RNA was quantified prior to initiation of antiviral therapy and at 12 weeks after the completion of antiviral therapy. Plasma levels of HCV RNA were quantified by the reverse transcriptase polymerase chain reaction (RT-PCR) method using the Abbott Real Time HCV Kit® with a detection range of 12–100,000,000IU/mL. A secondary endpoint was the assessment of liver function by comparing laboratory test and MELD scores at baseline and 12 weeks after completion of antiviral therapy.
2.3Safety assessmentDeath or hospitalization during treatment was considered a serious adverse event. The interruption or reduction of RBV associated to adverse events was recorded, as well as infections and haematological dyscrasias identified during treatment. Renal injury was defined as an increase in serum creatinine at the end of antiviral treatment equal to or greater than 50% of serum creatinine at the start of antiviral therapy.
2.4Statistical analysisAll patients that had received at least one dose of the previously described treatment regimens were included. We describe continuous variables by means and standard deviations (SD) or medians and interquartile ranges (IQR). We describe categorical variables by absolute and relative frequencies. Differences in baseline characteristics between the groups, grouped according to duration of therapy and use of RBV, were assessed using the Kruskall–Wallis test for continuous data and the chi-square test or the Fisher's exact test for the categorical tests. To compare changes in time in continuous variables, we used a paired t-test for normal distribution and the Wilcoxon signed-rank test for non-normal distribution variables. All p-values were two-sided tests and p<0.05 was considered statistically significant. All analyses were performed using STATA 12.0 software (StataCorp, College Station, Texas).
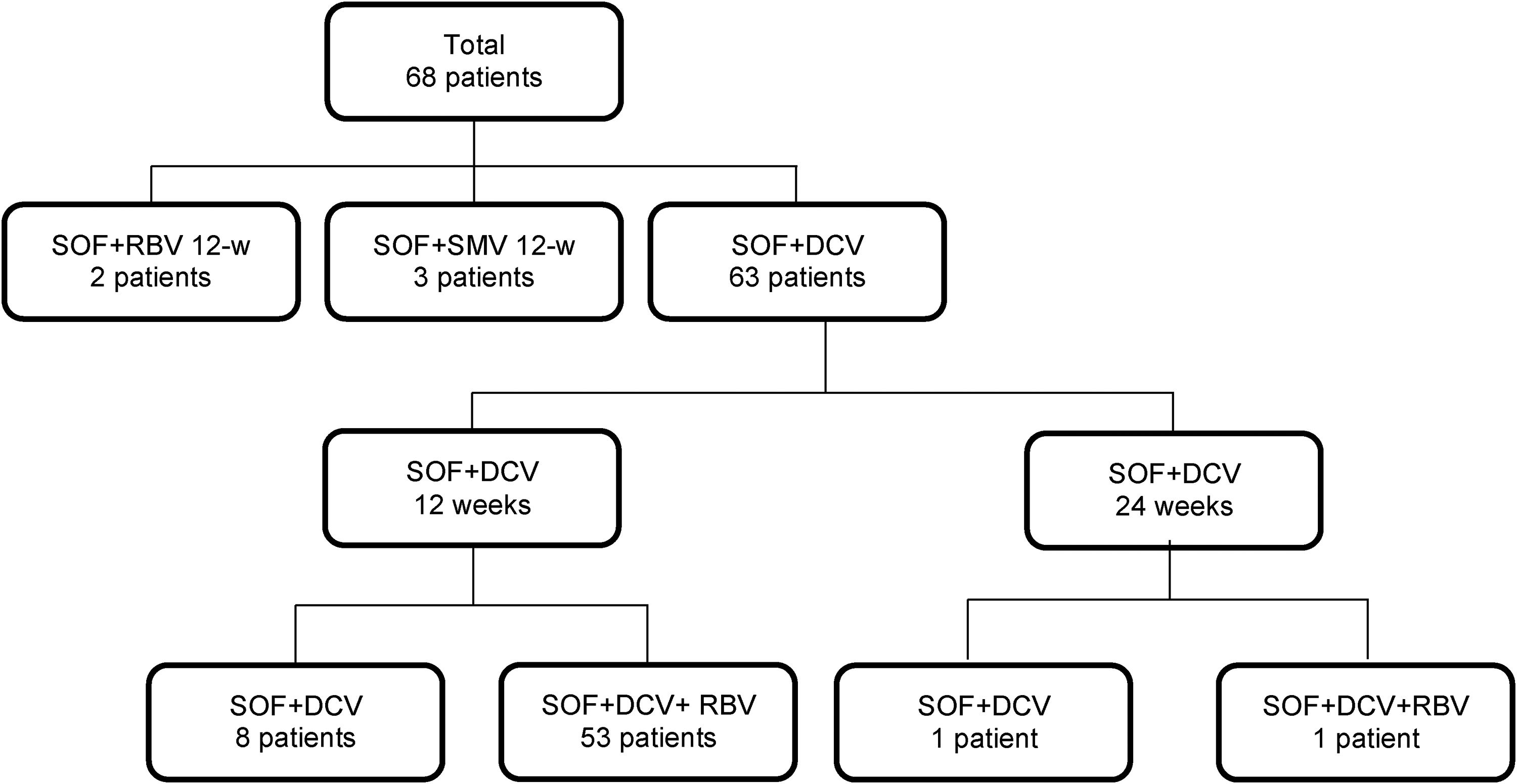
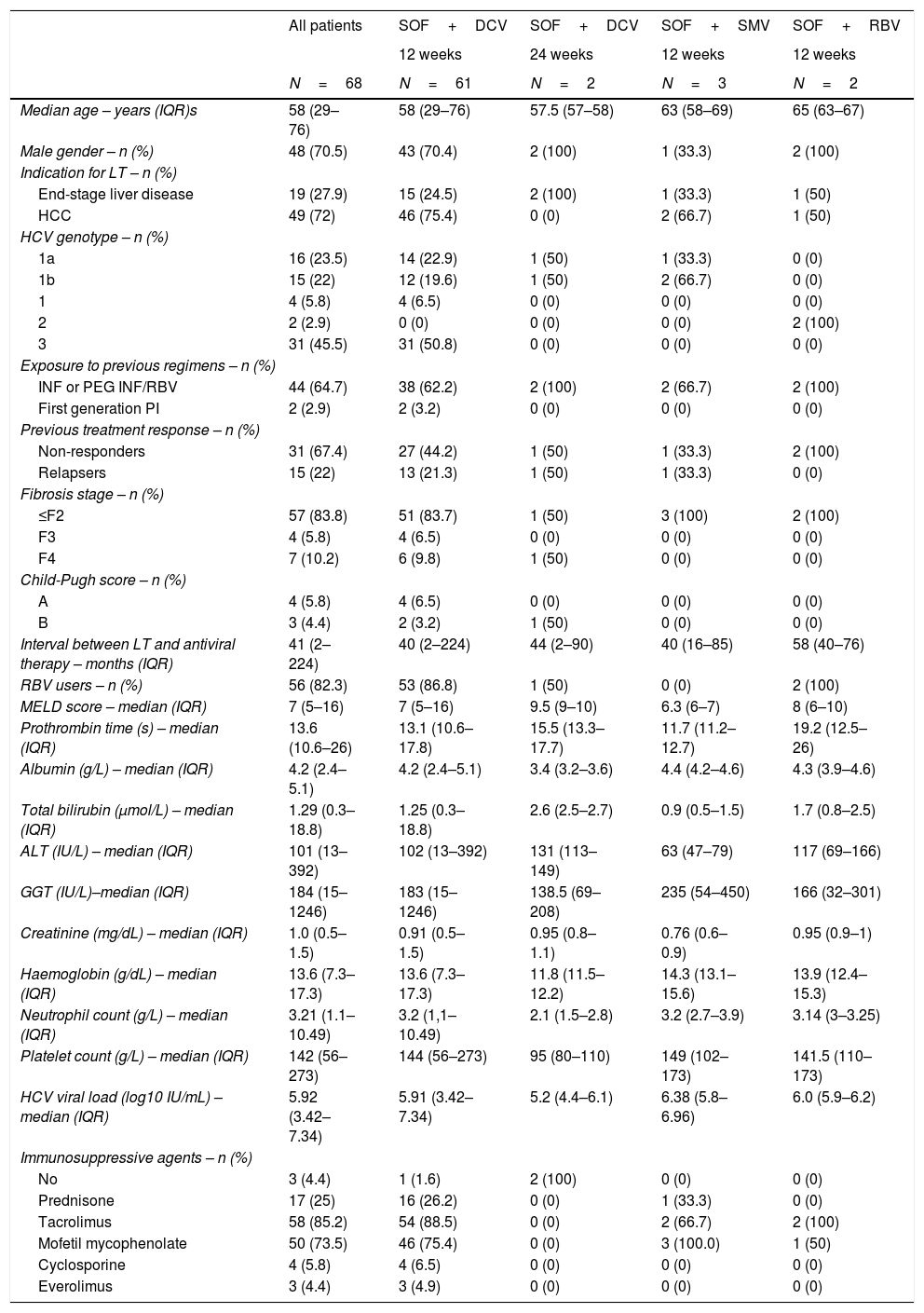
3Results3.1Clinical characteristics of the study populationSixty-eight patients with post-transplant recurrent HCV infection (70.6% were male, median age 58 years old, IQR 29–76) were treated with different SOF-based regimens between March 2015 and December 2016 (Fig. 1). The baseline characteristics of the patients included in the study are presented in Table 1. HCC was the major indication for LT (n=49, 72%).
Baseline characteristics of the study population.
| All patients | SOF+DCV | SOF+DCV | SOF+SMV | SOF+RBV | |
|---|---|---|---|---|---|
| 12 weeks | 24 weeks | 12 weeks | 12 weeks | ||
| N=68 | N=61 | N=2 | N=3 | N=2 | |
| Median age – years (IQR)s | 58 (29–76) | 58 (29–76) | 57.5 (57–58) | 63 (58–69) | 65 (63–67) |
| Male gender – n (%) | 48 (70.5) | 43 (70.4) | 2 (100) | 1 (33.3) | 2 (100) |
| Indication for LT – n (%) | |||||
| End-stage liver disease | 19 (27.9) | 15 (24.5) | 2 (100) | 1 (33.3) | 1 (50) |
| HCC | 49 (72) | 46 (75.4) | 0 (0) | 2 (66.7) | 1 (50) |
| HCV genotype – n (%) | |||||
| 1a | 16 (23.5) | 14 (22.9) | 1 (50) | 1 (33.3) | 0 (0) |
| 1b | 15 (22) | 12 (19.6) | 1 (50) | 2 (66.7) | 0 (0) |
| 1 | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 2 (2.9) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| 3 | 31 (45.5) | 31 (50.8) | 0 (0) | 0 (0) | 0 (0) |
| Exposure to previous regimens – n (%) | |||||
| INF or PEG INF/RBV | 44 (64.7) | 38 (62.2) | 2 (100) | 2 (66.7) | 2 (100) |
| First generation PI | 2 (2.9) | 2 (3.2) | 0 (0) | 0 (0) | 0 (0) |
| Previous treatment response – n (%) | |||||
| Non-responders | 31 (67.4) | 27 (44.2) | 1 (50) | 1 (33.3) | 2 (100) |
| Relapsers | 15 (22) | 13 (21.3) | 1 (50) | 1 (33.3) | 0 (0) |
| Fibrosis stage – n (%) | |||||
| ≤F2 | 57 (83.8) | 51 (83.7) | 1 (50) | 3 (100) | 2 (100) |
| F3 | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| F4 | 7 (10.2) | 6 (9.8) | 1 (50) | 0 (0) | 0 (0) |
| Child-Pugh score – n (%) | |||||
| A | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| B | 3 (4.4) | 2 (3.2) | 1 (50) | 0 (0) | 0 (0) |
| Interval between LT and antiviral therapy – months (IQR) | 41 (2–224) | 40 (2–224) | 44 (2–90) | 40 (16–85) | 58 (40–76) |
| RBV users – n (%) | 56 (82.3) | 53 (86.8) | 1 (50) | 0 (0) | 2 (100) |
| MELD score – median (IQR) | 7 (5–16) | 7 (5–16) | 9.5 (9–10) | 6.3 (6–7) | 8 (6–10) |
| Prothrombin time (s) – median (IQR) | 13.6 (10.6–26) | 13.1 (10.6–17.8) | 15.5 (13.3–17.7) | 11.7 (11.2–12.7) | 19.2 (12.5–26) |
| Albumin (g/L) – median (IQR) | 4.2 (2.4–5.1) | 4.2 (2.4–5.1) | 3.4 (3.2–3.6) | 4.4 (4.2–4.6) | 4.3 (3.9–4.6) |
| Total bilirubin (μmol/L) – median (IQR) | 1.29 (0.3–18.8) | 1.25 (0.3–18.8) | 2.6 (2.5–2.7) | 0.9 (0.5–1.5) | 1.7 (0.8–2.5) |
| ALT (IU/L) – median (IQR) | 101 (13–392) | 102 (13–392) | 131 (113–149) | 63 (47–79) | 117 (69–166) |
| GGT (IU/L)–median (IQR) | 184 (15–1246) | 183 (15–1246) | 138.5 (69–208) | 235 (54–450) | 166 (32–301) |
| Creatinine (mg/dL) – median (IQR) | 1.0 (0.5–1.5) | 0.91 (0.5–1.5) | 0.95 (0.8–1.1) | 0.76 (0.6–0.9) | 0.95 (0.9–1) |
| Haemoglobin (g/dL) – median (IQR) | 13.6 (7.3–17.3) | 13.6 (7.3–17.3) | 11.8 (11.5–12.2) | 14.3 (13.1–15.6) | 13.9 (12.4–15.3) |
| Neutrophil count (g/L) – median (IQR) | 3.21 (1.1–10.49) | 3.2 (1,1–10.49) | 2.1 (1.5–2.8) | 3.2 (2.7–3.9) | 3.14 (3–3.25) |
| Platelet count (g/L) – median (IQR) | 142 (56–273) | 144 (56–273) | 95 (80–110) | 149 (102–173) | 141.5 (110–173) |
| HCV viral load (log10 IU/mL) – median (IQR) | 5.92 (3.42–7.34) | 5.91 (3.42–7.34) | 5.2 (4.4–6.1) | 6.38 (5.8–6.96) | 6.0 (5.9–6.2) |
| Immunosuppressive agents – n (%) | |||||
| No | 3 (4.4) | 1 (1.6) | 2 (100) | 0 (0) | 0 (0) |
| Prednisone | 17 (25) | 16 (26.2) | 0 (0) | 1 (33.3) | 0 (0) |
| Tacrolimus | 58 (85.2) | 54 (88.5) | 0 (0) | 2 (66.7) | 2 (100) |
| Mofetil mycophenolate | 50 (73.5) | 46 (75.4) | 0 (0) | 3 (100.0) | 1 (50) |
| Cyclosporine | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Everolimus | 3 (4.4) | 3 (4.9) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: DCV: daclatasvir; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; INF: interferon; IQR: interquartile range LT: liver transplantation; M: male; n: number; PEG INF: pegylated interferon; PI: protease inhibitor; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir.
HCV genotype distribution was 51.4% (n=35), 3% (n=2) and 45.6% (n=31), for genotypes 1, 2 and 3, respectively. Of the 35 patients with genotype 1, 16 were genotype 1a (45.7%), 15 were genotype 1b (42.8%) and in 4 it was impossible to determine a subgenotype. Only 22 patients were treatment naïve (32.3%), and the majority of previously treated patients were non-responders (31/46, 67.4%). Significant fibrosis (Metavir F3) and cirrhosis were identified in 4 (5.8%) and 7 (10.2%) patients, respectively. The median time between liver transplantation and initiation of treatment was 41 (IQR 13–54) months. Sixty-one patients (89.7%) received SOF plus DCV for 12 weeks, two patients (2.9%) received SOF plus DCV for 24 weeks, three patients (4.4%) received SOF plus SMV for 12 weeks, two patients (2.9%) received SOF plus RBV for 12 weeks; 97% of the patients (66/68) underwent treatment for 12 weeks and 82.3% of patients (56/68) used RBV. The median MELD score was 7 points (IQR 6–8). During antiviral therapy, tacrolimus (n=58, 85.2%) and mofetil mycophenolate (n=50, 73.5%) were the most commonly used immunosuppressant drugs.
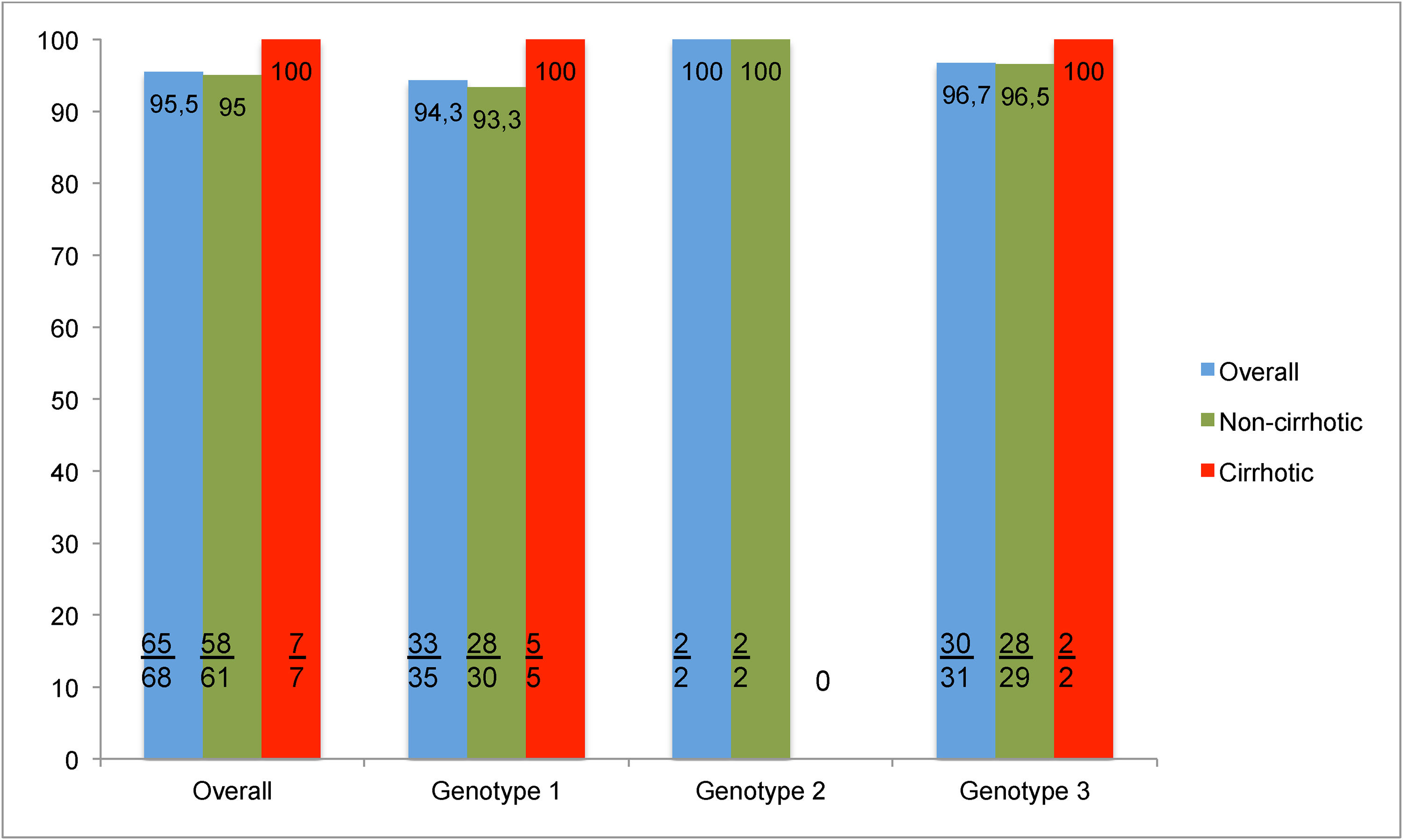
3.2Treatment efficacy and clinical improvementOverall SVR12 was 95.5% (65/68) (Fig. 2). Only 3 patients had virologic failure. All of whom were non-cirrhotic; with liver biopsy revealing fibrosis stage F2 in one patient and F0 in the other two; had HCV genotypes 3, 1a and 1b respectively, and were treated with SOF+DCV+RBV for 12 weeks. Resistance associated substitution analysis was not available for testing.
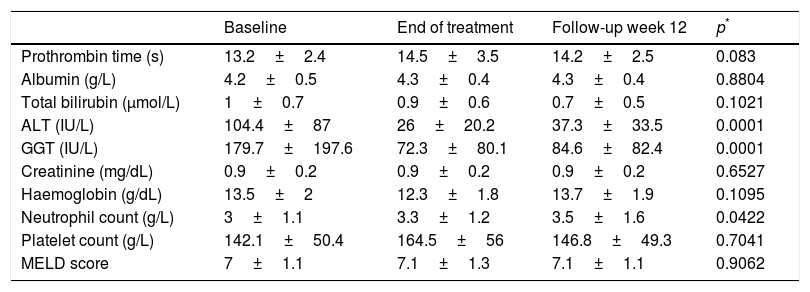
There was an improvement in ALT, GGT and neutrophil count during treatment but there was no significant variation in the other liver tests (Table 2).
Evolution of laboratory tests during the study.a
| Baseline | End of treatment | Follow-up week 12 | p* | |
|---|---|---|---|---|
| Prothrombin time (s) | 13.2±2.4 | 14.5±3.5 | 14.2±2.5 | 0.083 |
| Albumin (g/L) | 4.2±0.5 | 4.3±0.4 | 4.3±0.4 | 0.8804 |
| Total bilirubin (μmol/L) | 1±0.7 | 0.9±0.6 | 0.7±0.5 | 0.1021 |
| ALT (IU/L) | 104.4±87 | 26±20.2 | 37.3±33.5 | 0.0001 |
| GGT (IU/L) | 179.7±197.6 | 72.3±80.1 | 84.6±82.4 | 0.0001 |
| Creatinine (mg/dL) | 0.9±0.2 | 0.9±0.2 | 0.9±0.2 | 0.6527 |
| Haemoglobin (g/dL) | 13.5±2 | 12.3±1.8 | 13.7±1.9 | 0.1095 |
| Neutrophil count (g/L) | 3±1.1 | 3.3±1.2 | 3.5±1.6 | 0.0422 |
| Platelet count (g/L) | 142.1±50.4 | 164.5±56 | 146.8±49.3 | 0.7041 |
| MELD score | 7±1.1 | 7.1±1.3 | 7.1±1.1 | 0.9062 |
Abbreviations: ALT: alanine amino-transferase; GGT: gamma-glutamyl-transferase.
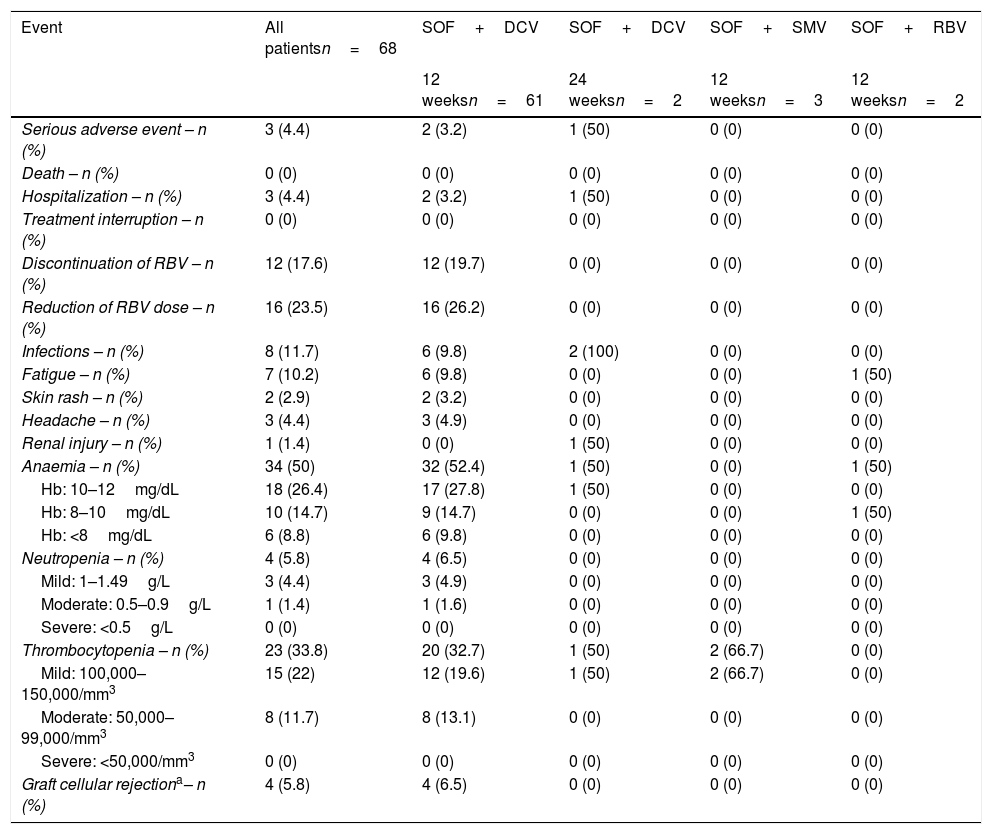
Most of the identified adverse events (AEs) were not serious (Table 3). Only three patients had serious AEs and hospitalization was necessary due to syncope associated with severe anaemia, urinary tract infection by multiresistant Escherichia coli and sepsis with no identified site. Anaemia was the most common AE (n=34, 5%) especially in patients who received RBV in comparison to those who did not (56.6% vs. 16.7%, p=0.012). Blood transfusion was required in only one case. None of the patients used erythropoietin (EPO). Other identified AEs were infection (n=8, 11.7%), fatigue (n=7, 10.2%), headache (n=3, 4.4%), skin rash (n=2, 2.9%) and renal injury (n=1, 1.4%). Urinary tract infection (4/7), sinusitis (2/7) and otitis (1/7) were identified. Also neutropenia and thrombocytopenia were observed in 4 (5.8%) and 23 (33.8%) patients, respectively, although none of the cases were severe.
Adverse events and laboratory abnormalities during treatment.
| Event | All patientsn=68 | SOF+DCV | SOF+DCV | SOF+SMV | SOF+RBV |
|---|---|---|---|---|---|
| 12 weeksn=61 | 24 weeksn=2 | 12 weeksn=3 | 12 weeksn=2 | ||
| Serious adverse event – n (%) | 3 (4.4) | 2 (3.2) | 1 (50) | 0 (0) | 0 (0) |
| Death – n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hospitalization – n (%) | 3 (4.4) | 2 (3.2) | 1 (50) | 0 (0) | 0 (0) |
| Treatment interruption – n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Discontinuation of RBV – n (%) | 12 (17.6) | 12 (19.7) | 0 (0) | 0 (0) | 0 (0) |
| Reduction of RBV dose – n (%) | 16 (23.5) | 16 (26.2) | 0 (0) | 0 (0) | 0 (0) |
| Infections – n (%) | 8 (11.7) | 6 (9.8) | 2 (100) | 0 (0) | 0 (0) |
| Fatigue – n (%) | 7 (10.2) | 6 (9.8) | 0 (0) | 0 (0) | 1 (50) |
| Skin rash – n (%) | 2 (2.9) | 2 (3.2) | 0 (0) | 0 (0) | 0 (0) |
| Headache – n (%) | 3 (4.4) | 3 (4.9) | 0 (0) | 0 (0) | 0 (0) |
| Renal injury – n (%) | 1 (1.4) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Anaemia – n (%) | 34 (50) | 32 (52.4) | 1 (50) | 0 (0) | 1 (50) |
| Hb: 10–12mg/dL | 18 (26.4) | 17 (27.8) | 1 (50) | 0 (0) | 0 (0) |
| Hb: 8–10mg/dL | 10 (14.7) | 9 (14.7) | 0 (0) | 0 (0) | 1 (50) |
| Hb: <8mg/dL | 6 (8.8) | 6 (9.8) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia – n (%) | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Mild: 1–1.49g/L | 3 (4.4) | 3 (4.9) | 0 (0) | 0 (0) | 0 (0) |
| Moderate: 0.5–0.9g/L | 1 (1.4) | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) |
| Severe: <0.5g/L | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thrombocytopenia – n (%) | 23 (33.8) | 20 (32.7) | 1 (50) | 2 (66.7) | 0 (0) |
| Mild: 100,000–150,000/mm3 | 15 (22) | 12 (19.6) | 1 (50) | 2 (66.7) | 0 (0) |
| Moderate: 50,000–99,000/mm3 | 8 (11.7) | 8 (13.1) | 0 (0) | 0 (0) | 0 (0) |
| Severe: <50,000/mm3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Graft cellular rejectiona– n (%) | 4 (5.8) | 4 (6.5) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: Hb: haemoglobin, DCV: daclatasvir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir.
Four patients (5.8%) presented severe cellular graft rejection after HCV treatment. All the patients were using tacrolimus at low doses; two of them were using mofetil mycophenolate and only one patient was using prednisone. Three patients recovered with increasing doses of immunosuppressive therapy and one of them evolved to chronic rejection.
4DiscussionDAA therapy revolutionized the management of HCV after LT, enabling early post-LT treatment with high rates of SVR12, low drug-drug interactions and good tolerability. Furthermore, the clearance of HCV prevents evolution to end-stage liver disease at the graft. The majority of studies reported low rates of adverse events in patients who treated HCV with DAA after liver transplantation.
This real-life study describes the experience of DAA treatment of recurrent HCV infection after LT in a public university hospital in the south of Brazil. This is the first report of SOF-based therapy after liver transplantation in Latin America. We use different SOF-based regimens, with or without RBV, for 12 or 24 weeks. The highest proportion of patients had HCV genotypes 1 and 3. About 10% of patients had cirrhosis. No patient with HCC after liver transplant was included. SOF+DCV+RBV for 12 weeks was the most widely used treatment regimen. Very few patients were treated with SOF+SMV or SOF+RBV, hence no comparison of the effectiveness of the therapies was performed. The overall SVR12 was 95.5% (65/68 patients). Three patients had virologic failure. Surprisingly, they were non-cirrhotic (two patients with fibrosis staging F0 and one patient with fibrosis staging F2) with HCV genotypes 1b, 1a and 3 respectively, and were treated with SOF+DCV+RBV for 12 weeks. All patients with cirrhosis had SVR12 including two patients with decompensated cirrhosis. The identified AEs were associated with the use of RBV and the immunosuppression status of the study population. Anaemia was the most common AE (n=34, 50%), which led to RBV dose reduction (n=16) or withdrawal (n=12). Infection was the second most frequent AE (11.7%). Few patients required hospitalization for AE treatment.
The results presented here are in full accordance with other clinical trials and real-life studies. Castells et al. [22] evaluated 22 liver transplant recipients with HCV recurrence, 69% treatment experienced, 56% with cirrhosis, 27% decompensated, treated with SOF+DCV for 24 weeks. All 22 patients achieved SVR12. Coilly et al. [21] conducted a multicentre cohort study using DCV plus SOF to treat recurrent HCV after liver transplant (CUPILT Study). The authors included 137 patients, 33% with cirrhosis, the majority being genotype 1 (79%). The SVR12 was 96% and 99% when excluding non-virologic failures. Only two patients experienced a virologic failure. The serious adverse events (SAE) rate was 17.5%. Maria et al. [23] reported the real life experience of HCV treatment after liver transplantation in Sweden. The authors included 93 patients with HCV genotypes 1, 2, 3 or 4 (58, 7.5, 26.5 and 7.5%, respectively), treated with SOF in combination with SMV or NS5A inhibitor (DCV or ledipasvir-LDV), with or without RBV, or SOF plus RBV for 12 or 24 weeks. SVR12 was 97.8% (91/93 patients). Qu et al. [24] conducted a systematic review to assess the efficacy and safety of SOF-based therapy in liver transplantation recipients with recurrent HCV and included 22 studies (1730 patients). The pooled SVR12 was 90.1%. Another systematic review and meta-analysis was reported by Liao et al. [25], including 7 studies with 379 liver transplant recipients with recurrent HCV treated with SOF plus DCV. Most of the patients had genotype 1 HCV infection. The overall rate of SVR12 was 93.3%. The most common adverse events were anaemia (32%), infections (26%) and neutropenia (23%).
Our study found similar results, with an overall SVR12 >95% and good therapy tolerability, using SOF-based regimens, mainly SOF+DCV+RBV for 12 weeks. However, we observed 4 patients who developed severe graft rejection after HCV elimination. So far, severe rejection after HCV therapy has not been reported in previous studies. All these patients had stable immunosuppression. Interestingly, resuming steroids, the use of high doses of tacrolimus, mycophenolate or everolimus was needed for the treatment of such severe rejection. One patient showed no improvement despite high doses of immunosuppressant drugs, and evolved to chronic graft rejection. Saxena et al. [26] recently reported the safety and efficacy of DAA therapy in Kidney and Liver Transplant recipients with hepatitis C in the HCV-TARGET Study. The authors included 443 patients: 60 kidney transplant (KT), 347 liver transplant (LT) and 36 liver-kidney transplant (LKT), 42% with cirrhosis and 54% treatment experienced, most had HCV genotype 1 (87%). The treatment regimens used were SOF+LDV±RBV (85%), SOF+DCV±RBV (9%) and ombitasvir/paritaprevir/ritonavir+dasabuvir±RBV (6%). SVR12 was 95.7% (96.3%, 94.6% and 90.9% among LT, KT and LKT recipients, respectively). Six episodes of acute rejection occurred (1.4%): 2 in KT and 4 in LT recipients. The rate of acute rejection was similar to that found in patients after transplant who are not treating HCV. The causality of acute rejection was not directly attributed to HCV therapy. In the interferon era, graft rejection was associated with the immunomodulatory effect of interferon or the improvement in hepatic function after HCV clearance, and better pharmacokinetics of immunosuppressant drugs leading to lower trough levels. In the interferon-free era, some immunologic changes can be observed after HCV treatment. Martin et al. [27] described the restoration of T-cell function induced by Interferon-free therapy. The authors demonstrated the lymphocytic exhaustion hypothesis induced by the persistent HCV antigenic exposure, which could be restored after HCV clearance with DAA therapy. This form of immune reconstitution could be the trigger to T-lymphocyte mediated graft rejection.
The key limitations of this study are inherent to its retrospective design, the small number of patients with cirrhosis (7 patients, 10.2%) and the high frequency of RBV use (56 patients, 82.3%), limiting the ability to assess the influence of RBV on efficacy and safety.
In conclusion, DAA therapy based in SOF is highly effective and safe for the treatment of HCV after LT. Our study detected severe graft rejection in some patients after HCV clearance, which may be related to T-lymphocyte immune reconstitution. However, further studies are needed to establish the real risk of graft rejection after HCV cure.
AbbreviationsAASLD American Association for the Study of Liver Diseases adverse event colony forming units daclatasvir erythropoietin Fundo de Incentivo a pesquisa hepatocellular carcinoma hepatitis C virus Hospital de Clínicas de Porto Alegre interferon liver transplant model for end-stage liver disease peginterferon ribavirin serious adverse event simeprevir sofosbuvir sustained virologic response ribonucleic acid reverse transcriptase polymerase chain reaction Unified Health System World Organization of Gastroenterology
This study has received no financial support.
Author's contributionsAlexandre Araujo, Vanessa Valenzuela-Granados, Antonio B. Lopes, Matheus T. Michalczuk and Mario R. Álvares-da-Silva contributed substantially to the conception and design of the paper.
Alexandre Araujo, Vanessa Valenzuela-Granados, Antonio B. Lopes, Matheus T. Michalczuk, Augusto Mantovani collected and analyzed the data.
Alexandre Araujo, Antonio B. Lopes, Mario R. Álvares-da-Silva drafted and critically revised the paper for important intellectual content.
All the authors authorized the submission for publication of the final version of the paper.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to thank all the patients and their families for their support in the development of this study. Likewise, we thank the World Gastroenterology Organization of (WGO) and the Professional Team of the WGO Porto Alegre Hepatology Training Centre for establishing opportunities for the training of young gastroenterologists in the area of Hepatology and Investigation.