Background/Purpose. Hepatitis C (HCV) is a major cause of morbidity and mortality in haemophilia patients who received clotting factor concentrates before the availability of virus-inactivated factors in the mid-1980s. Recently, it has been suggested that anti-HCV treated patients, particularly those achieving a sustained virological response (SVR) have an improved outcome. We sought to examine the survival of treated and untreated HCV-infected haemophilia patients.
Material and methods. We studied overall and liver-related survival of patients with haemophilia and other congenital bleeding disorders between 2000 and 2010. The outcome was compared in 3 sub-groups: HCV mono-infected (N = 127), HCV/HIV co-infected (N = 28), and patients with either HCV-antibodies negative or persistent HCV RNA-negative (referred to as non-infected) (N = 45). Sixty-two (40%) (HCV and HCV/HIV) patients underwent anti-HCV treatment with an SVR rate of 40.3%.
Results. Overall and liver-related 10-year survival were: 82.1 and 89.3%, 95.3 and 99.2 and 100% for HCV/HIV co-infected, HCV mono-infected and non-infected haemophilia patients, respectively (p = 0.015 and 0.023 for comparisons of HCV/HIV vs. HCV; p = 0.003 for comparison of HCV/HIV and non-infected). One HCV mono-infected and 3 co-infected patients died of end-stage liver disease (2 underwent liver transplantation). There was no survival benefit from anti-HCV treatment or from attaining of an SVR. Only clinically suspected cirrhosis remained as an independent predictor of survival.
Conclusion. The prognosis of haemophilia patients who acquired HCV/HIV co-infection is worse than that of HCV mono-infected or non-infected or haemophiliacs. This is mainly due to liver-related mortality. Anti-HCV treatment or SVR had no observable impact on survival rate.
Chronic hepatitis C (HCV) infection has a high prevalence in haemophilia patients (ranging between 40-90%), as well as in other hereditary bleeding disorders consequent upon receipt of pooled non virusinactivated clotting factor concentrates during the 1970s and early 1980s.1–3 In this regard, transmission of HCV from factor concentrates prior to routine viral attenuation has been considered to be almost universal.1 Of all infected patients, between 10 and 20% will spontaneously clear the virus with the generation of measurable anti-HCV antibodies and with persistently negative HCV RNA levels.
Within the HCV-infected group, a significant number of haemophilia patients became co-infected with both HCV and HIV where many of these patients previously succumbed to AIDS prior to the era of effective antiretroviral therapy.2,3 With the wider use of highly active antiretroviral therapy (HAART), the prognosis of HIV infection has substantially improved so that HCV infection has assumed a much greater clinical importance, where liver disease has become the major cause of morbidity and mortality.4 Furthermore, co-infection with HIV virus is recognized as an independent predictor of liver disease progression. Amongst those with HCV/HIV co-infection, there is some evidence that HAART slows the progression to end stage liver disease (ESLD),5,6 although this remains controversial.7
We have estimated that patients with haemophila and other congenital coagulation disorders followed at the Israeli National Hemophilia Center (INHC) are now infected for about 25 years; therefore, about 20 to 30% of these patients have so far developed progressive fibrosis or cirrhosis.8–10
In those HCV-infected patients, the principal aim of anti-HCV treatment is to eradicate the virus and prevent disease progression, with assessment of the sustained virological response (SVR) which is defined as absence of viremia 24 weeks after cessation of all antiviral medication. Several studies demonstrated that SVR is associated with a reduced occurrence of liver failure and liver-related deaths in patients with chronic HCV and advanced hepatic fibrosis.11–15 However, data regarding the most substantial clinical end point i.e., improved survival and its relationship of SVR is scarce. Recently, it has been shown that the beneficial effects of SVR also result in reduced all-cause mortality in the high risk population of patients with chronic HCV infection and severe hepatic fibrosis.16 The strongest evidence on the association between virologic and overall survival is a large Veterans Affairs cohort study that found SVR to be associated with a 30 to 50% reduction in mortality risk, after adjustment for many confounders.17
Essentially all HCV/HIV co-infected patients at the INHC are treated with HAART regimens. Since 2000 all HCV-infected patient with haemophilia and other hereditary bleeding disorders were systematically evaluated for pegylated interferon alfa and ribavirin (PEG-IFN/RBV) combination therapy.
The aim of this study was to examine the overall and liver-related survival in a cohort of HCV-infected patients at our institution over a 10-year period. We also sought to investigate whether anti-HCV treatment and attaining an SVR would improve survival rates in these patients.
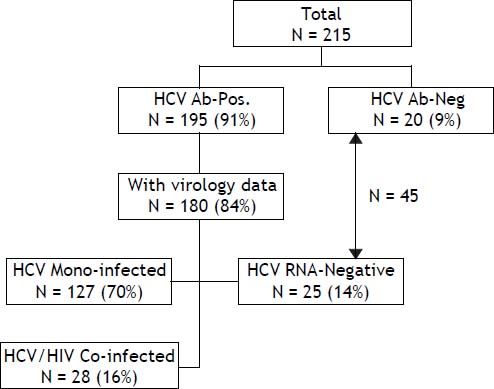
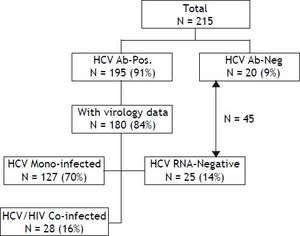
Material and MethodsSelection of patientsAbout 215 patients with haemophilia and other disorders of coagulation in Israel were born prior to 1986, so that they potentially were exposed to non-virucidally treated concentrated clotting factors for at least one year. All of these patients were managed at the INHC. Of this cohort, 195 (90.7%) tested positive for anti-HCV antibodies with a viral status being known in 180 (83.7%) patients. These patients were separated into 3 main groups: HCV mono-infected patients (N = 127), HCV/HIV co-infected patients (N = 28) and patients who tested positive for HCV-serology but who were persistently HCV RNA negative (N = 25) and who were considered to have spontaneously cleared their HCV infection (Figure 1). Patients with genotype 1 (and all genotypes in the HCV/HIV co-infected group) were treated with PEG-IFN/RBV for 48 weeks. Those patients with genotypes 2 and 3 were treated in the same way, although only for a 24 week period. Only patients who received at least 80% of the recommended PEG-IFN/RBV dose were considered evaluable for their response to treatment. Before commencing the PEG-IFN/RBV combination, the HAART regimen was individually modified in order to avoid adverse side-effects and undesirable drug interactions with Ribavirin, where Zidovudine and Didanosine were generally replaced with other antiretroviral medications.
For the purpose of determining non-responders, those patients were divided into 3 groups. Null-responders were defined as those who received at least 80% of the recommended PEG-IFN/RBV dose for at least 12 weeks but who achieved a < 2 log10 IU/mL reduction in HCV RNA serum levels when compared with their baseline. Partial responders were those who exhibited a ≥ 2 log10 IU/mL reduction of their viral load but who did not reach undetectable levels at any measurement time-point. Relapsers were defined as those who achieved undetectable HCV RNA serum levels at the end of the scheduled 24 or 48 weeks of PEG-IFN/RBV treatment but who had detectable HCV RNA levels within 24 weeks of follow-up. Sustained viral response (SVR) was defined as an undetectable HCV RNA level in the serum 24 weeks after treatment termination.
Clinical suspected cirrhosis was defined as the development of one of the following characteristics; including decompensated liver disease (ascites, hepatic encephalopathy, jaundice or bleeding varices), signs of hypersplenism (both an enlarged spleen and thrombocytopenia), ultrasonography suggestive of the presence of cirrhosis or any of its complications, or signs of portal hypertension (varices on upper gastrointestinal endoscopy or diagnostic 99Tc liver/ spleen scan). All patients who were diagnosed with clinical features consistent with cirrhosis were also staged as F4 on the Fibro-Test (FT). Calculation of the FT score was derived from serum samples measuring α2-macroglobulin, total bilirubin, γ-glutamyl trans-peptidase (GGT), apolipoprotein A1 and haptoglobin with adjustment according to the prescribed formula for age and gender.18 Total bilirubin, GGT and AST levels were measured by an Hitachi 917 Analyzer using Roche Diagnostics reagents (Mannheim, Germany) whereas α2-macroglobulin, apolipoprotein A1, haptoglobin and total cholesterol levels were measured using a Modular analyzer (BNII; Dade Behring, Marburg, Germany). All biochemical analyses were performed on serum samples shipped to the New Rambam Laboratory (Jerusalem, Israel) within a 2 h period. The FT score was calculated using the Biopredictive websiste according to the manufacturer’s instructions.18 FT determination was deferred during episodes of active haematoma, infection or inflammation in order to avoid misleading measurements of bilirubin, haptoglobin, and a2-macroglobulin.
Overall and liver-related survivalOverall and liver-related survival of HCV-infected and non-infected haemophilia patients and those with other congenital coagulation disorders all managed at a single center were recorded between 2000 and 2010. The cause of death was obtained from hospital records. Death was classified as liver related if the patient had cirrhosis and died from typical complications of terminal liver disease (variceal bleeding, failure of hepatic biosynthetic function, cholestasis, portal hypertension with ascites, hepatorenal syndrome, hepatic encephalopathy with coma), or had liver cancer. Liver transplantation was considered in the liver-related mortality category. The outcome of patients with haemophilia was compared in 3 main sub-groups: HCV mono-infected patients (N = 127), HCV/HIV co-infected patients (N = 28) and a third group which combined those patients who tested HCV-antibody negative (N = 20) with those who were persistently HCV RNA-negative (N = 25). This latter combined group of 45 patients was classified as non-infected. Haemophilia patients who tested positive for HCV-antibodies but where data concerning virological status was lacking (N = 15), were excluded from analysis. All patients belonging to this last group survived during the follow-up period (Figure 1).
Variables potentially influencing overall survival were analyzed including basic demographic data, characteristics of the underlying coagulation disorder, the presence of HCV/HIV co-infection, clinical features consistent with cirrhosis, the viral load (where a high viral load was defined as an HCV RNA level ≥ 800,000 lU/mL), the viral genotype and the stage of liver fibrosis as determined at baseline by the FT. The influence of anti-HCV treatment with the PEG-IFN/RBV combination on the SVR and the effect of SVR on survival were determined.
Statistical analysisQuantitative variables were expressed as means ± SD unless otherwise specified. Continuous variables were compared using the Student t-test with frequencies being compared utilizing the two-tailed Fisher’s exact test. For the purposes of analysis, null responders, partial responders and relapsers were all considered as one group. For the calculation of the age-at-infection and its duration, we applied a method of estimation based upon the haemophilia type, the severity of the disorder and the history of clotting factor replacement as previously described.19 Survival was analyzed with the Kaplan-Meier method with comparison between subgroups and those receiving anti-HCV treatment or having an SVR being performed with the log-rank test. Variables on study entry were assessed by univariate analysis (ANOVA) and were entered step-wise into a Cox proportional hazard regression model if a difference with a p-value of < 0.1 was detected. P-values of < 0.05 were considered significant. Statistical analysis was conducted using Statistical Analysis Software version 8.2 (SAS Institute Inc., Cary, NC).
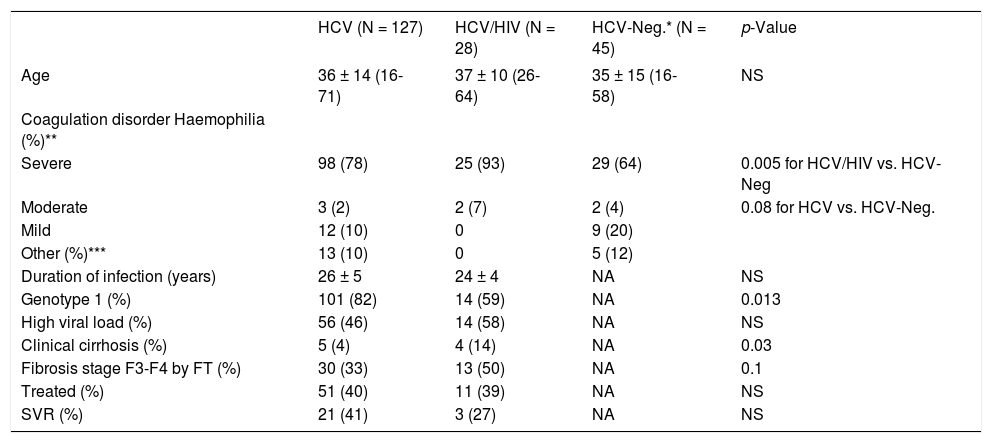
ResultsPatients characteristicsBaseline demographic, clinical and virological data are shown in Table 1. Both HCV/HIV co-infected and HCV mono-infected patients had a higher incidence of underlying severe haemophilia when compared with non-infected patients (93, 78 and 64%, respectively), with significance shown between HCV/HIV co-infected when compared with HCV-negative patients (p = 0.005). The estimated duration of HCV infection was about 25 years for both groups. Infection with the HCV genotype 1 was more prevalent in HCV mono-infected patients than in the HCV/HIV co-infected group (82 vs. 59%; p = 0.013). There was no statistically significant difference in the frequency of high viral load (i.e., HCV RNA ≥ 800,000 IU/mL) between HCV/HIV co-infected and HCV mono-infected patients (58 vs. 46%). Liver disease was more advanced in the HCV/HIV co-infected patients when compared with the HCV mono-infected patients as indicated by the presence of clinically suspected cirrhosis (14 vs. 4%; p = 0.03) and by an FT staging of F3 or F4, although this difference did not reach statistical significance (50 vs. 33%, respectively; p = 0.1).
Demographic, clinical, and virological data of haemophilia patients born before 1986.
| HCV (N = 127) | HCV/HIV (N = 28) | HCV-Neg.* (N = 45) | p-Value | |
|---|---|---|---|---|
| Age | 36 ± 14 (16-71) | 37 ± 10 (26-64) | 35 ± 15 (16-58) | NS |
| Coagulation disorder Haemophilia (%)** | ||||
| Severe | 98 (78) | 25 (93) | 29 (64) | 0.005 for HCV/HIV vs. HCV-Neg |
| Moderate | 3 (2) | 2 (7) | 2 (4) | 0.08 for HCV vs. HCV-Neg. |
| Mild | 12 (10) | 0 | 9 (20) | |
| Other (%)*** | 13 (10) | 0 | 5 (12) | |
| Duration of infection (years) | 26 ± 5 | 24 ± 4 | NA | NS |
| Genotype 1 (%) | 101 (82) | 14 (59) | NA | 0.013 |
| High viral load (%) | 56 (46) | 14 (58) | NA | NS |
| Clinical cirrhosis (%) | 5 (4) | 4 (14) | NA | 0.03 |
| Fibrosis stage F3-F4 by FT (%) | 30 (33) | 13 (50) | NA | 0.1 |
| Treated (%) | 51 (40) | 11 (39) | NA | NS |
| SVR (%) | 21 (41) | 3 (27) | NA | NS |
Table 1 depicts the characteristics of the HIV infection in those haemophilia patients with HCV/HIV co-infection. All except one patient were treated with HAART combinations, where most of them attained satisfactory immunological control (mean CD4 + count 382 ± 207 cells/mm3), and low or undetectable (62% of co-infected patients) HIV viral load.
Overall, 62 patients (40%) received anti-HCV treatment during the study period, including 51/127 (40.2%) of the HCV mono-infected patients and 11/ 28 (39.3%) of the HCV/HIV co-infected group. All these patients were treated between 2001 and 2003. An SVR (as defined) was observed overall in 25/62 (40.3%) of the treated patients; with an SVR in 22/ 51 (43.1%) of the HCV mono-infected and in 3/11 (27.3%) of the HCV/HIV co-infected patients. A fibrosis stage of F3 or F4 determined by the FT was present in 49.1% of the treated patients vs. 25.8% of those not treated (p = 0.009).
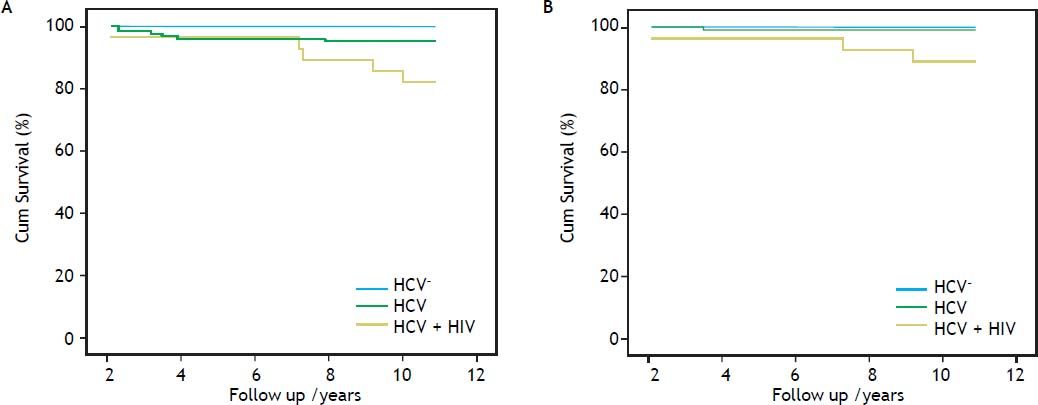
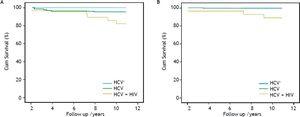
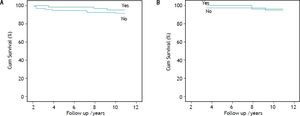
Overall and liver-related survivalComparative cumulative overall and liver-related survival over a 10 year follow-up are shown in figure 2. The overall survival of the HCV/HIV co-infected patients was inferior to that of both the HCV mono-infected and the non-infected patients (81.2, 95.3 and 100%, respectively), showing significance for HCV/HIV vs. HCV mono-infected patients (p = 0.015) and in comparison of HCV/HIV co-infected vs. non-infected patients (p = 0.003). The overall survival rate of the HCV mono-infected patients did not significantly differ from the non-infected group (p = 0.14). The liver-related survival of the HCV/HIV co-infected patients was lower when compared with HCV mono-infected patients (89.2 vs. 99.8%; p = 0.023) and when compared with non-infected patients (89.2 vs. 100%; p = 0.003), where no significant difference was noted in overall survival between the HCV mono-infected and the non-infected patients (p = 0.55).
Overall (A) and liver-related (B) cumulative survival of hepatitis C-infected haemophilia patients (Overall survival: HCV/HIV vs. HCV; p = 0.015; HCV/HIV vs. non-HCV; p = 0.003; HCV vs. non-HCV; p = 0.14) (Liver-related Survival: HCV/HIV vs. HCV; p = 0.023; HCV/HIV vs. non-HCV; p = 0.003; HCV vs. non-HCV; p = 0.55).
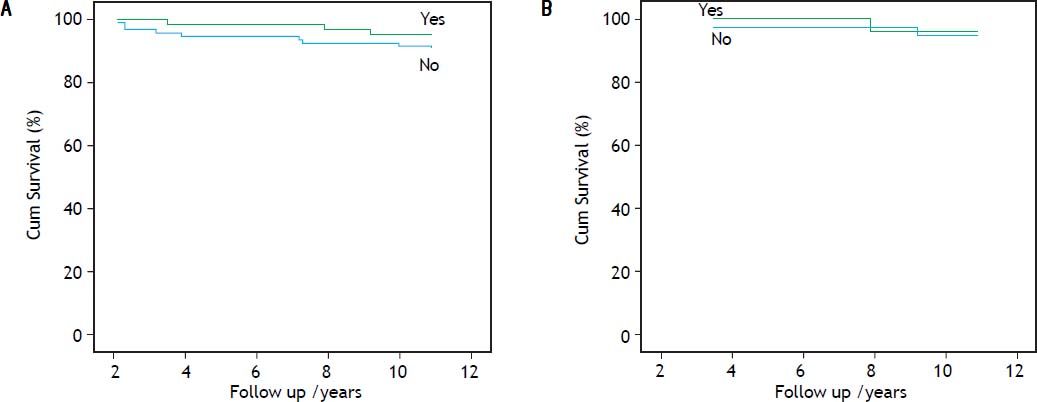
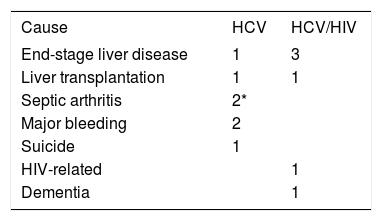
The causes of mortality in 11 patients who died between 2000 and 2010 are shown in table 2 with the major cause of death in the HCV/HIV co-infected patients being liver-related. One patient underwent combined liver-kidney transplantation and is still alive. Amongst the HCV mono-infected patients, a further patient underwent liver transplantation for ESLD but died in the immediate postoperative period. Of the 62 patients who received anti-HCV treatment 59 (95.2%) survived, while of the 93 untreated patients 85 (91.4%) survived (p = 0.37).
Cause of death in hepatitis C-infected haemophilia patients.
| Cause | HCV | HCV/HIV |
|---|---|---|
| End-stage liver disease | 1 | 3 |
| Liver transplantation | 1 | 1 |
| Septic arthritis | 2* | |
| Major bleeding | 2 | |
| Suicide | 1 | |
| HIV-related | 1 | |
| Dementia | 1 |
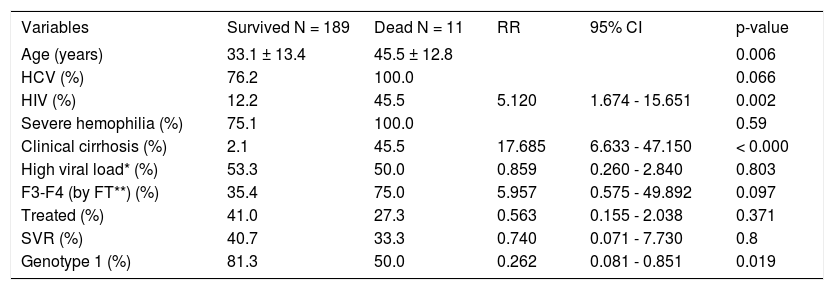
Table 3 shows the results of the univariate and multivariate analyses, respectively incorporating factors which predicted for overall survival. On univariate analysis, advanced age, HCV/HIV co-infection, coincident clinically suspected cirrhosis and a non-1 genotype infection acted as predictive factors for mortality, however, neither anti-HCV treatment with the PEG-IFN/RBV combination nor the development of an SVR (which occurred in 25 patients), influenced survival. On multivariate analysis, only clinically suspected cirrhosis remained as an independent predictor of overall mortality (OR = 15.119; 95% CI, 2.388-95.735; p = 0.004) (Table 3). The effects of anti-HCV treatment and the presence of an SVR on cumulative overall survival are shown in figure 3, respectively.
Clinical and virological variables predicting survival-Univariate analysis.
| Variables | Survived N = 189 | Dead N = 11 | RR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age (years) | 33.1 ± 13.4 | 45.5 ± 12.8 | 0.006 | ||
| HCV (%) | 76.2 | 100.0 | 0.066 | ||
| HIV (%) | 12.2 | 45.5 | 5.120 | 1.674 - 15.651 | 0.002 |
| Severe hemophilia (%) | 75.1 | 100.0 | 0.59 | ||
| Clinical cirrhosis (%) | 2.1 | 45.5 | 17.685 | 6.633 - 47.150 | < 0.000 |
| High viral load* (%) | 53.3 | 50.0 | 0.859 | 0.260 - 2.840 | 0.803 |
| F3-F4 (by FT**) (%) | 35.4 | 75.0 | 5.957 | 0.575 - 49.892 | 0.097 |
| Treated (%) | 41.0 | 27.3 | 0.563 | 0.155 - 2.038 | 0.371 |
| SVR (%) | 40.7 | 33.3 | 0.740 | 0.071 - 7.730 | 0.8 |
| Genotype 1 (%) | 81.3 | 50.0 | 0.262 | 0.081 - 0.851 | 0.019 |
Our study found a higher overall and liver-related mortality in an Israeli cohort of haemophilia patients born prior to 1986 who contracted combined HCV and HIV infection when compared with HCV mono-infected and non-infected patients. The main cause of death in HCV/HIV co-infected patients was ESLD. Although HIV infection was fairly well controlled with the HAART regimens in essentially all patients, their unfavorable outcome persisted. There was no effect either of the use of anti-HCV therapy (combined PEG-IFN/RBV) or if an SVR has been attained with treatment. Only the presence of clinically suspected cirrhosis remained as an independent negative prognostic variable of survival.
Our report has several limitations. First, this is a retrospective analysis, nevertheless, the collection of data was based on regular follow-up of patients with haemophilia and other disorders of coagulation all managed at one center — the INHC. Given this very special population, our cohort is conceivably relatively small. Nonetheless, the prevalence of HCV infection is very high among haemophilia patients compared with other populations at risk to contract such infection.
Combination therapy with HAART regimens can be limited in some cases by hepatotoxicity, increase in HCV viral load and enhanced fibrosis progression rate.7 However, control of HIV replication has been associated with a delay in the progression of hepatic fibrosis, a longer time to initial hepatic decompensation and a prolonged overall survival. In this respect, long-term follow-up from a German haemophilia cohort5 has shown that effective antiretroviral treatment lowers the mortality in patients with HCV-related chronic liver disease, where the peak of liver-related mortality was found between 1991 and 1996, after approximately 10 years of recorded co-infection and prior to the introduction of effective antiretroviral therapy. In this study, the mortality rate then declined from 1997 until 2002, suggesting a selective survival benefit in co-infected patients being treated with HAART. Another study from several US haemophilia centers,6 reported that the median ESLD-free survival was significantly shorter in HIV-positive than HIV-negative men with haemophilia. In contrast, ESLD-free survival among HAART-treated HIV-infected men was significantly longer than in non-HAART-treated HIV-infected men 30 vs. 20 year, but was similar to that in HIV-negative men.
In our study, essentially all HCV/HIV co-infected haemophilia patients were treated with HAART that induced an acceptable immunological and virological control. Over a 10-year follow-up period we found 19 and 11% overall and liver-related mortality rates, respectively. In contrast to the aforementioned reports, mortality rates were still significantly higher in HCV/HIV co-infected patients when compared with HCV mono-infected (5 and 1%, respectively) or non-infected patients (none). Nevertheless, even though mortality is greater, the mortality in HIV/ HCV is still relatively low over 10 years.
Prior studies in patients with HCV and severe hepatic fibrosis have reported reduction in complications of advanced liver disease and improved liver-related mortality associated with SVR.11–15 Most studies however, did not investigate all-cause mortality as a single outcome, and reduction in liver-related death may not directly translate into an overall survival benefit. In a recent international, multicenter, long-term follow-up study,16 SVR was associated with prolonged overall survival. This study with a long follow-up duration demonstrated a lower risk for all-cause mortality in patients with chronic HCV infection and advanced hepatic fibrosis who achieved SVR. The risk of all-cause mortality was almost 4-fold lower in patients with SVR compared with patients without SVR. In another study in patients with advanced fibrosis or cirrhosis, the association of SVR with all cause death and liver transplantation as a combined end point was analyzed.15 The adjusted cumulative proportion of patients who died or underwent liver transplantation after 7.5 years of follow-up was higher in patients not responding to PEG-IFN/RBV therapy (27.2%) compared with patients with virological relapse (4.4%) or who achieved an SVR (2.2%). In the largest study, predominantly male population of US veterans was followed up for a median of 3.8 years. In this cohort with all stages of liver fibrosis, 5-year mortality rates of 6.7 to 8.0% in patients with SVR vs. rates of 14.4 to 24.4% in patients without SVR were reported.17
Regression of fibrosis as assessed by paired liver biopsies was reported in non-haemophilic HCV patients who achieved an SVR.20 A study from a Dutch haemophilia cohort21 demonstrated that even after a long HCV infection duration, successful antiviral treatment led to a significant improvement of fibrosis, evaluated by liver stiffness measurement. It is anticipated that the objective attenuation of fibrosis may select for survival in those patients who develop an SVR.
To our knowledge, the present report is the first attempt to correlate anti-HCV treatment and SVR with survival in a cohort of haemophilia patients. A principal question remains as to why in our cohort, overall or liver-related survival benefit was not observed following anti-HCV treatment and why there was no clinical effect even in those who achieved an SVR. The relatively small number of patients may not allow for a sufficient statistical power to show such a relationship. Furthermore, in previous reports survival advantage was demonstarated only in those patients achieving an SVR. The SVR rate in our cohort was lower than the SVR rates reported in other HCV-infected haemophiliacs or non-haemophilic populations.22–25 We also acknowledge some bias caused by inadvertent selection of patients with more advanced stage of fibrosis to undergo anti-HCV treatment. Indeed, advanced fibrosis as assessed by the FT was observed in about a half of the treated patients, whereas it was found in only a quarter of the untreated group. Finally, most studies have shown both an overall and liver-related survival advantage in patients with advanced fibrosis or cirrhosis capable of generating an SVR, although there are confounding variables which also limit the interpretation of comparative data. Only one study including over 16,000 patients showed such advantage in a population representing all stages of liver fibrosis.17 In this particular study only 9 to 16% of the included patients were registered as having cirrhosis, nevertheless, the relatively high death rate in the study of the US veterans may be due to other co-morbidities in this particular patient population.
In summary, in our population of HCV-infected patients with haemophilia and other congenital bleeding disorders, we found an unfavorable outcome in HCV/HIV co-infected patients mainly due to complications of advanced liver disease. This outcome was not improved by anti-HCV treatment even when it resulted in an SVR. As treatment failure remains the main challenge for HCV mono-infected and HCV/HIV co-infected patients, there may be future promise in the use of novel directly acting drugs (DDI) that may also benefit those who fail previous standard of care or who display specific predictors of poor treatment outcome.26
AddendumY. Maor-Conceived and designed the study, interpreted and analyzed the data, wrote the manuscript and gave final approval of the version to be published.
J.M. Schapiro-Designed the study, interpreted the data, critically revised the intellectual content and gave final approval of the version to be published.
D. Bashari-Designed and generated the study database, critically revised the intellectual content and gave final approval of the version to be published.
U. Martinowitz-Conceived and designed the study, interpreted the data, critically revised the intellectual content and gave final approval of the version to be published.
Disclosures of Conflict of InterestThe authors declare no conflict of interest during the conduct of this trial.
Compliance with Ethical RequirementsAll the authors hereby state that they do not have any conflict of interest/financial disclosure. The study was approved by the local IRB committee. According to national regulations retrospective studies do not require signed informed consent from patients. The study does not involve animals.
AcknowledgementsMedical writing support was commissioned from A. Zbar CEO surgical-steps.com