The main objectives of the treatment of chronic hepatitis C are eradication of the virus and reduction of symptoms to avoid progression to cirrhosis and its complications and development of hepatocarcinoma.1-3 Treatment should not be restricted to patients with favorable response profiles; all patients with hepatitis C should be considered for treatment. Decisions on the selection of patients for treatment should be based on case histories, disease progression rates, comorbidities, risk-benefit ratios, and cost-effect factors.2
Chronic hepatitis C is associated with nonspecific symptoms such as fatigue, lethargy, and abdominal pain, the extent of which is not correlated with the severity of liver damage. The mere presence of these symptoms does not constitute an indication for treatment.3,4 General indications for initiation of treatment include:
a) hypertransaminasemia (> 1.5 times normal values for more than 6 months),
b) positive serology for hepatitis C virus (HCV) and PCR-detectable HCV RNA, and
c) mild to severe chronic hepatitis evident in a liver biopsy (Metavir score > 2 or mild to severe necroinflammatory activity).5-7
In patients with elevated levels of transaminase and mild hepatitis (Metavir score < 2 and light necroinflammatory activity), the need for treatment should be evaluated on an individual basis, taking into account factors such as extrahepatic symptoms and manifestations, comorbid conditions that increase the risk of progression to fibrosis (e.g., obesity and coinfection with HIV), HCV genotypes 2 and 3, (high probability of a response), patients with primoinfection, and patients wishing to conceive.5,6
Treatment regimens depend on the type of interferon administered and the viral genotype present. Pegylated interferon alfa-2a (PEG-IFN alfa-2a) (40KD) is administered as a single weekly dose of 180 μg, while pegylated interferon alfa-2b (PEG-IFN alfa-2b) is administered in weekly doses of 1.5 μg/kg body weight. The duration of treatment and the dose of ribavirin depend on the viral genotype. Patients with HCV genotypes 1, 4, or 5 require treatment for 48 weeks with high doses of ribavirin (1000 mg/d for body weight < 75 kg; 1200 mg/d for bodyweight > 75 kg Patients with HCV genotypes 2 and 3 require treatment for 24 weeks with low doses of ribavirin (800 mg/d). However, when ribavirin is administered with PEG-IFN alfa-2b, the recommended daily dose of ribavirin is 10.6 mg/kg bodyweight.1,5,8-11
The best index of the effectiveness of treatments for hepatitis C is the sustained viral response (SVR, absence of HCV RNA 48 weeks after conclusion of treatment). However, viremia during week 12 of treatment is a good indicator of sustained viral response, particularly in patients with genotype 1 HCV. In this group, patients who have negative or even positive SVRs and significant reductions of viral loads (> 2 log10) should either be treated for 48 weeks or withdrawn from treatment because they have a low probability of attaining a sustained viral response (SVR). Patients with genotypes 4, 5, and 6 should be treated according to the regime used for genotype 1 patients. It is not necessary to control viremia for longer than 12 weeks in patients with genotypes 2 and 3, as regardless of its outcome, and because of the high response rate, the treatment will be maintained during the established 24 weeks4,12(Figure 1).
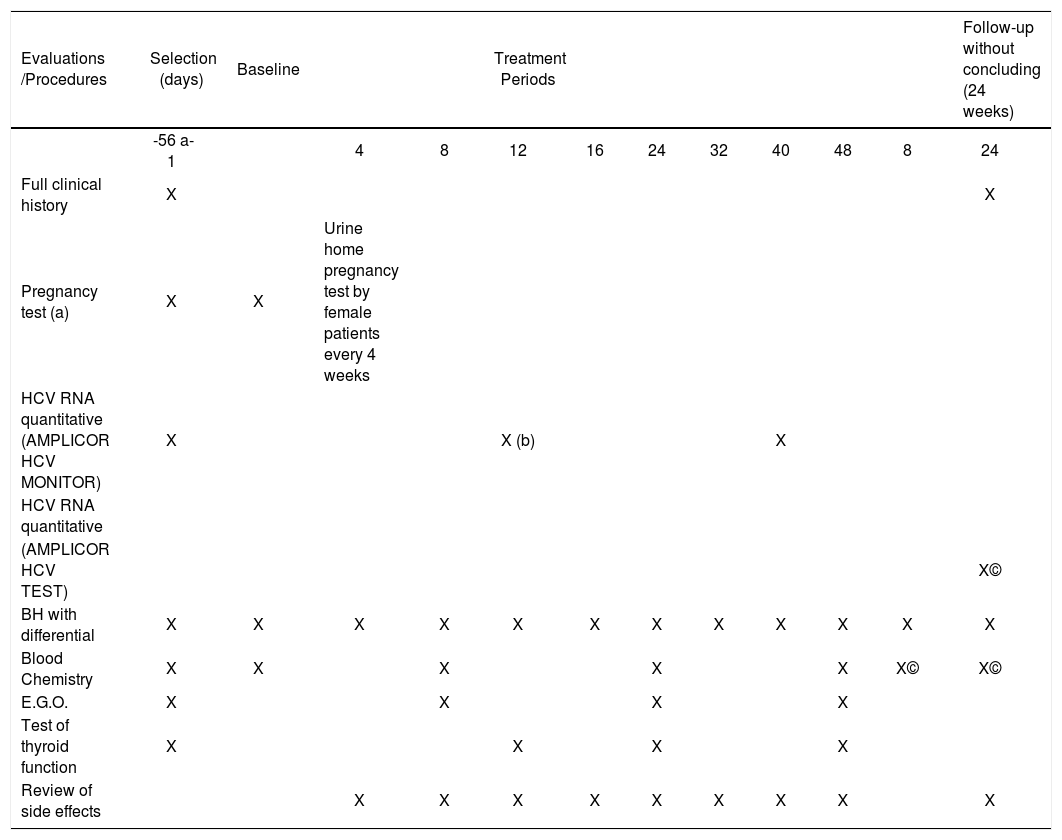
Treatment with interferon and ribavirin is not free of side effects, which include flu-like symptoms, headache, fever, and myalgias in up to 50% of patients 6 to 8 hours after the initial injection. Many of the side effects are dose dependent and do not require discontinuation of treatment. About 1%–2% of patients present with serious adverse effects including exacerbation of preexisting psychiatric disease, liver failure, and thyroid malfunction. Ribavirin may cause hemolytic anemia in 10% of patients.1,5,9,12 Patients should be monitored closely once treatment is initiated (Table I).
Evaluation and follow-up program
| Evaluations /Procedures | Selection (days) | Baseline | Treatment Periods | Follow-up without concluding (24 weeks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -56 a-1 | 4 | 8 | 12 | 16 | 24 | 32 | 40 | 48 | 8 | 24 | ||
| Full clinical history | X | X | ||||||||||
| Pregnancy test (a) | X | X | Urine home pregnancy test by female patients every 4 weeks | |||||||||
| HCV RNA quantitative (AMPLICOR HCV MONITOR) | X | X (b) | X | |||||||||
| HCV RNA quantitative | ||||||||||||
| (AMPLICOR HCV TEST) | X© | |||||||||||
| BH with differential | X | X | X | X | X | X | X | X | X | X | X | X |
| Blood Chemistry | X | X | X | X | X | X© | X© | |||||
| E.G.O. | X | X | X | X | ||||||||
| Test of thyroid function | X | X | X | X | ||||||||
| Review of side effects | X | X | X | X | X | X | X | X | X |
Recommendations of the consensus panel
1. What is the best therapy for treatment-naïve patients?
Therapy with pegylated interferon plus ribavirin is recommended for 48 weeks for patients with HCV genotype 1 and for 24 weeks for patients with other genotypes. Follow-up visits should be conducted every 15 days during the first 2 months and on a monthly basis thereafter. However, departures from the scheduled program of visits may be necessary because of the personal circumstances of individual patients.
Evidence quality: 1