The diagnostic utilities of ultrasonography (US), fatty liver index (FLI) and an algorithm of nine serum markers (Fibromax) were evaluated in family practice to noninvasively characterize patients with nonalcoholic fatty liver disease (NAFLD). A multicenter study was conducted by enrolling 259 consecutively observed patients (age 51 ± 10 years) with clinical and ultrasonographic features of NAFLD. Patients had mild (16.2%), moderate (69.9%), or severe (13.9%) liver steatosis and 60.2% had hypertransaminasemia. The percent of patients with overweight, obesity, diabetes, hypertension, and dyslipidemia were 42.7%, 46.5% (4.2% severe obesity), 24.7%, 40.9%, and 56.4%, respectively. Lean patients (10.8%) had normal transaminases in two/ thirds of the cases. A multivariate logistic regression (including age > 50 yrs, BMI > 30 kg/m2, HOMA > 3, and hypertransaminasemia) identified 12.3% of patients at risk for steatohepatitis. With a sensitivity of 50% and specificity of 94.7%, Fibromax identified 34 patients (13.1%) with likely advanced fibrosis and found that over 28% of patients with moderate (ultrasonographic) steatosis were likely to be carrying severe steatosis. Steatotest score was significantly associated with BMI, waist circumference, ALT, triglycerides, and FLI. Fibrotest correlated only with ALT. FLI identified 73.4% of patients as likely to be carrying a fatty liver. In conclusion, NAFLD should be systematically searched and characterized in all patients with metabolic disturbances and cardiovascular risk. Asymptomatic subjects at risk also should be screened for NAFLD. Fibromax is a promising noninvasive diagnostic tool in family medicine for identifying patients at risk for NAFLD who require targeted follow-up.
Nonalcoholic fatty liver disease (NAFLD) is a clinico-histopathological entity with histological features resembling alcohol-induced liver injury. NAFLD occurs in patients with negligible or negative history of alcohol consumption. Histologically, the spectrum of NAFLD ranges from fat accumulation in hepatocytes without concomitant inflammation or fibrosis (simple hepatic steatosis) to hepatic steatosis with a necroinflammatory component (steatohe-patitis) with or without fibrosis (nonalcoholic steatohepatitis, NASH). NAFLD is an emerging problem in westernized societies with high impact on care utilization and costs1 and is frequently associated with the metabolic syndrome and cardiovascular diseases.2–4 Prevention and early identification of NASH is of key importance since, whereas “simple” steatosis is benign, NASH puts 5-8% of patients at risk of cryptogenic cirrhosis within 5 years.5,6
The ultimate diagnosis of NASH is based on liver biopsy, an invasive procedure not free of complications and poorly accepted by patients. Liver biopsy, moreover, is not currently advisable for the large scale population and carries potential bias including sampling error and intra –and inter– observer discrepancies.7 Therefore, noninvasive tests are actively being investigated and are useful in family practice to select the subset of patients requiring further consultations.8
A number of score systems are currently available to predict chronic liver disease noninvasively, but none is ideal. Scores provide the best information on the likelihood of having steatosis (fatty liver index, FLI) or an already established cirrhosis (AST/platelet ratio, APRI), but they lack sensitivity in the intermediate forms of liver disease.9,10 The identification of NASH represents a major task for general practitioners (GPs) who likely meet this problem at an early stage when hypertransaminasemia and ultrasonography (US) are of little help.11
Most data on NAFLD derive from a secondary or even tertiary level on already selected patients, and very few data come from family practice which handles more than half of primary health care and outpatient services.12 Also, most epidemiological studies have provided data on the prevalence of NAFLD in at risk populations (obese, diabetics, dyslipidemics),13 and conversely few data are available on the characterization of these patients. This study aimed to evaluate liver steatosis and disease staging by noninvasive scores and to define in the family practice the general health status of patients with NAFLD.
Experimental ProceduresA multicenter national study named “VARES” was entirely conducted in a family medicine setting within the Italian College of General Practitioners (SIMG). One practitioner was coordinating4–8 investigators who had been previously trained on the problem of NAFLD. Each practitioner agreed to enroll 10 consecutive patients (18-65 years old) from which history, US, and clinical features of NAFLD were collected. From April to October 2011, 259 patients (165 males, age 51 ± 10 years) were enrolled; 23 lean matched healthy subjects served as controls.
Standard measurements included height, weight, and body mass index (BMI) expressed as body weight (in kg) divided by the height (in meters) squared. Subjects were classified as normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese class I (30-34.9 kg/m2), and obese class II-III (> 35 kg/m2). Waist circumference, as a marker of visceral adiposity, was measured with a flexible tape placed on a horizontal plane at the level of the iliac crest. According to the ATPIII criteria, values were abnormal if ≥ 88 cm and ≥ 102 cm in females and males, respectively. Patients were excluded if their histories suggested alcohol ingestion, and indeed, alcohol ingestion was either absent or < 10 g/day in all subjects. Patients with other chronic liver diseases (virus, transition metal accumulation, autoimmune, genetic disorders, and liver cirrhosis) or with one of the following conditions were also excluded: history of recent acute or chronic hemolysis, acute hepatitis, acute inflammation or concomitant bacterial or viral infection, extra hepatic cholestasis, Gilbert’s disease, protease inhibitor therapy, and dialysis.
Steatosis at US was semiquantitatively assessed according to a previously reported scale:14 i.e., 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Other information included duration of hypertransaminasemia (at least twice over the previous 24 months with levels exceeding 30 IU/mL for males and 20 IU/mL for females). If present, gallstones, diabetes, arterial hypertension, metabolic syndrome, coronary heart disease, chronic renal failure, and chronic drug intake were noted.
Venous blood was obtained from overnight fasting subjects and used to measure serum insulin, transaminases, cholesterol, triglycerides, and the other parameters to calculate the Fibromax algorithm (haptoglobin, bilirubin, gamma-GT, A1-apolipoprotein, alpha2-macroglobulin) (www.biopredictive.it). This test provides information on liver fatty infiltration scored 0 to 3 (Steatotest), grade of inflammation scored absent, borderline, present (NASHTest), and fibrosis scored 0 to 4 (Fibrotest). Insulin resistance was calculated by the HOMA formula. FLI was calculated by an algorithm including serum triglycerides, gammaGT, BMI, and waist circumference.9
During the six months following the end of the study and out of the study protocol, 16 hypertransaminasemic patients underwent liver biopsy based on the specialist’s decision. All subjects joined the study by giving their written consents; the protocol was approved by the Ethical Committee of Bari University Hospital (Italy) and respected the Declaration of Helsinki.
Statistical analysisData are given as mean and standard error (SEM). A power of the study test (alpha value set at 0.05 and desired power 0.80) was performed with the Fibrotest to identify the minimum significant number of patients potentially carrying a liver fibrosis: result indicates n = 245. Results were analyzed for statistical difference by the Student’s t test for unpaired data, the Chi-square, and linear regression. To identify subclasses of patients, the effects of anthropometric and metabolic parameters on Fibromax scores were calculated as coefficients in a retrospective model of multivariate logistic regression with subject groups (absent versus present) as independent factors. The sensitivity and specificity of a single test (Fibrotest and Steatotest), positive and negative predictive values were calculated to differentiate patients with high grade of fibrosis or severe steatosis from those with no/low fibrosis or mild steatosis.
ResultsMetabolic profileHypercholesterolemia was found in 119 patients (45.9%) with 20.1% of those patients on statins. Arterial hypertension was found in 106 (40.9%), hypertriglyceridemia in 81 (31.3%), metabolic syndrome in 77 (29.7%), diabetes in 63 (24.3%), gallstones in 28 (10.8%), coronary heart disease in 9 (3.5%), and chronic renal failure in 3 (1.1%). Hypertransaminasemia was found in 156 patients (60.2%).
Overall, BMI was 30 ± 5 kg/m2, and waist circumference was 103 ± 11 cm in females and 104 ± 11 cm in males. According to BMI, patients were normal weight (n = 28, 10.8%), overweight (n = 109, 42.7%), and obese (n = 122, 46.5%): class I (n = 111, i.e. 42.3% of total), class II (n = 11, i.e. 4.2% of total). Lean patients had mild (n = 12), moderate (n = 15), and severe (n = 1) liver steatosis at US. Of them, 15 (53.6%) had normal transaminase levels, 18 (64.3%) hypercholesterolemia, and 11 (39%) were under steatogenic drugs. Conversely, a severe liver steatosis was found in n = 19 moderate obese and in n = 3 severe obese patients.
Liver profileAccording to US images, 42 patients (16.2%) carried a mild steatosis, 181 (69.9%) had moderate steatosis, and 36 (13.9%) had severe steatosis (Figure 1). Within the group of severe steatosis, 6 (16.7%) patients had normal transaminase levels (Table 1). According to FLI, 190 (73.4%) patients showed a high likelihood of having a fatty liver, 54 (20.8%) patients were borderline, and 15 (5.8%) patients were unlikely to have a fatty liver.
Anthropometric and clinical features alterations in patients with nonalcoholic fatty liver disease (n=259). Patients were divided in three subgroups according to ultrasonography.
| Ultrasonography | Normal weight | Overweight | Moderate obesity | Severe obesity | Elevated transaminases | Arterial hypertension | Diabetes lipid | High |
|---|---|---|---|---|---|---|---|---|
| Mild steatosis (n = 42) | 42.8 | 27.4 | 18.4 | 11.4 | 43 | 31 | 24 | 24 |
| Moderate steatosis (n = 181) | 33.6 | 30.7 | 31.8 | 3.6 | 60 | 41 | 24 | 52 |
| Severe steatosis (n = 36) | 3.6 | 21.9 | 47.2 | 27.3 | 83 | 50 | 25 | 83 |
Data are reported as percentage of patients included in each subgroup.
The results of the Steatotest showed that 76 patients (29.4%) were “S0-S1” (absence or mild fatty infiltration), 63 (24.3%) were “S2” (moderate steatosis), and 120 (46.3%) were “S3” (severe steatosis). The NASH test results were low in 52 (20.1%) patients, borderline in 190 (73.3%) patients, and high in 17 (6.6%) patients. The Fibrotest showed that 120 patients (46.3%) were “F0-F1” (absence or minimal fibrosis), 105 patients (40.6%) were “F1-F2” (mild-moderate fibrosis), and 34 patients (13.1%) were “F3-F4” (important fibrosis) (Figure 1). Nine out 34 (26.5%) patients with F3-F4 had normal ALT levels.
In figure 2A patients were stratified according to US, FLI and Steatotest scores. In subjects with mild steatosis at US, FLI was highly likely (n = 20), uncertain (n = 16) and unlikely (n = 6), while according to Steatotest, the same patients fell into S0-S1 (n = 16), S2 (n = 12), and S3 (n = 14). Patients with moderate steatosis at US had a FLI highly likely (n = 143), uncertain (n = 31) and unlikely (n = 7); the same patients were S0-S1 (n = 50), S2 (n = 61), and S3 (n = 70) at Steatotest. Patients carrying a severe steatosis at US were all FLI highly likely; at Steatotest, we found S0-S1 (n = 1), S2 (n = 7) and S3 (n = 28) (Figure 2B).
Distribution of patients according to the different methods used to quantify liver steatosis. A. Relationship between the probability of having a fatty liver (Fatty liver index, FLI) and the Steatotest score (S0-S1 = 0-5%, mild; S2 = 6-32%, moderate; S3 ≥ 32%, severe fatty infiltration). Different symbols identified patients according to the extent of fatty infiltration at ultrasonography: □= mild, ○ = moderate, ▼ = severe steatosis. B. Patients distribution according to the Steatotest score within the different grades of liver steatosis at ultrasonography.
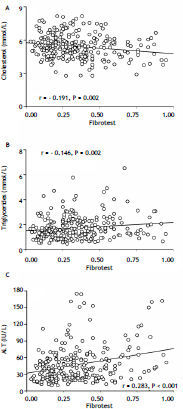
Steatotest values were significantly associated with BMI (r = 0.503), waist circumference (r = 0.412), HOMA (r = 0.259), serum triglycerides (r = 0.392), and ALT (r = 0.454) (Figure 3), but not with cholesterol (r = 0.089) or with Fibrotest (r = 0.049). Fibrotest scores were significantly related to cholesterol (r = -0.191), triglycerides (r = 0.146), and ALT (r = 0.283) (Figure 4).
By multivariate logistic regression (age > 50 years, diabetes, obesity, elevated transaminases) (13), n = 26 (10%) patients were highly suspected to carry a NASH with fibrosis. By considering waist circumference instead of BMI, the number raised to 29 (11.2%).
Considering an alpha value of 0.05 and a sample size of 282 subjects, Fibrotest was able to significantly (P = 0.01, power ANOVA = 0.801) discriminate within NAFLD patients according to fibrosis stages. In particular, NAFLD patients with F 3-4 were significantly different from healthy controls and patients with F 0-2. The regression analysis showed age > 50 years, diabetes, elevated transaminases, and waist circumference or obesity, expressed as BMI, as highly significant and independent factors for the observed differences in Fibrotest scores. With a cut-off of 0.37, the sensitivity of the test in identifying patients with advanced fibrosis (F 3-4) was 50% while the specificity was 94.7%, with a positive predictive value of 0.90 and negative predictive value of 0.73.
On the same subjects, Steatotest significantly (P = 0.001, power ANOVA = 0.892) discriminated within NAFLD patients according to the extent of fatty infiltration. In particular, NAFLD patients with S3 significantly differed from healthy subjects and patients with S0-2. The sensitivity of the test to identify patients with severe steatosis (S3) was 77% and the specificity was 88% with a positive predictive value of 0.92 and a negative predictive value of 0.80.
Liver biopsySixteen patients with Fibrotest scores of F3-F4 (12 S3 and 4 S2 at Steatotest, 8 with moderate and 8 severe steatosis at US, all FLI high probable) underwent liver biopsy. Histology was performed on liver specimens and reported according to Brunt, et al.:15 pictures showed n = 14 stage 3 and n = 2 stage 2 fibrosis, n = 10 severe and n = 6 moderate steatosis, grade 3 inflammation in all of them.
DiscussionThe prevalence of NAFLD in Italy and in Western countries is about 20-40% in the general population and even as much as 70-80% in obese and diabetic subjects.16–19 NASH has a prevalence of 2-3% in lean subjects,20 about 20% in obese, and 50% in morbidly obese patients.21
The diagnosis of NAFLD in family practice remains presumptive because it often lacks histology. With a sensitivity and a specificity of 89% and 93% respectively, US is currently accepted as the best method to screen for liver steatosis.22 However, US poorly defines the grade of fatty infiltration (by our data 30% of S3 patients at Steatotest were mild/moderate at US), is poorly accurate for diagnosing fibrosis (sensibility 77%, specificity 89%), and does not identify NASH (AUROC 0.65).23
By multivariate logistic regression and Fibrotest, over 11% of our NAFLD patients may unknowingly carry a NASH; these patients have a chronic disease and need consultation. These data differ from the USA study which showed a high prevalence of NASH (30% of NAFLD in about 12% of the general population).
By analyzing the FibroMax data, it appears that all mild steatotic patients (S0-S1) were negative for NASHtest and Fibrotest and 80% of them had normal transaminases; whereas, 85% of severely steatotic subjects had elevated transaminases although NASHtest and Fibrotest were normal or mildly altered. This indicates, as also suggested by animal studies, that the extent of fatty infiltration would not be determinant for inflammation and fibrosis.24 Moreover, 30% of patients likely to be carrying an important fibrosis (F3-F4) had normal transaminase levels. Therefore, in agreement with previous histologic results,25,26 ALT levels seem to discriminate the extent of steatosis but not of fibrosis. Finally, both BMI and serum triglycerides may affect the extent of steatosis but are not predictors of fibrosis.
Fibrotest was useful for staging fibrosis in HCV patients and has been proposed instead of liver biopsy in most of these patients. Our study, although lacking histology in most patients, confirms the utility of FibroMax to stage NAFLD patients. Potential application may consider the association with other non-invasive assessments (i.e. elastometry, breath tests).27 In this view, the specific role of GPs relies on early diagnosis for a better prognosis and, thus, for lowering the costs of the patient’s management. GPs have the competence to manage uncomplicated forms of NAFLD by empowering patients in diagnostic and therapeutic choices. In the scenario of exponential increase of diabetes and obesity, the validation of noninvasive biomarkers may have important implication for screening NAFLD/NASH and allowing GPs to spread true information regard-ing NAFLD. In this view, our data confirm a valid association between NAFLD and cardiovascular risk factors which show a higher prevalence than in the general population. By comparing our data with those recently reported in a similar USA population in which liver biopsy was performed in about 50% of patients,28 differences were noted for arterial hypertension (40.9 vs. 50%), metabolic syndrome (30 vs. 60%), diabetes (24.7 vs. 16.5%), and dyslipidemia, especially in lean subjects. Indeed, NAFLD should be searched also in lean patients with risk factors, since over 15% of them may likely carry a severe steatosis even with normal transaminases levels (50% of cases).
Finally, although it has been reported that the gallstones prevalence in NAFLD patients is higher than in the general population,29 we found data similar to those regarding the general population30 and even a lower prevalence in severe steatotic patients. This observation may pave the way to further investigations.
ConclusionFibroMax emerges as a promising noninvasive and easy tool to evaluate liver steatosis and disease staging and to identify patients to refer for further treatment? NAFLD has to be searched in patients, including lean subjects,with cardio-metabolic risk factorsand GPs play a key role in this respect.
AcknowledgementsThe following GPs actively participated in the study project: Abbruzzese Isabella, Albanese Pietro, Ardito Dionisio, Bocchino Giancarlo, Ciaccia Angela, D’Ambrosio Gaetano, Dell’Orco Mario, Laringe Matteo, Mastronuzzi Tecla, Morelli Lucia, Pasculli Domenico, Ramunni Angelo, Salvia Antonio, Scardino Maria Lucia, Scotti Aldo, Simonetti Maria Teresa, Viola Dario, Zamparella Maria. The authors are indebted to Ms. Diana Schaubhut for skillful language assistance.
Conflict of InterestAll authors declare that they have no relationships with any company that may have an interest in the submitted work and have no financial interests that may be relevant to the submitted work.
Financial SupportAll authors are independent from funders.
Abbreviations- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: nonalcoholic steatohepatitis.
- •
BMI: body mass index.
- •
FLI: fatty liver index.
- •
APRI: AST/platelet ratio.
- •
ALT: alanine aminotransferase.
- •
GPs: general practitioners.
- •
US: ultrasonography.
- •
SIMG: Italian College of General Practitioners.
- •
HOMA: homeostasis model assessment.
- •
CT: computerized tomography.