Anastomotic leak (AL) is a serious complication in colorectal surgery due to its increase in morbidity and mortality. The aim of this prospective non-randomised study is to determine whether C-reactive Protein (CRP) is useful as a predictor of AL in patients undergoing open versus laparoscopic surgery.
MethodsA total of 168 patients undergoing elective colorectal surgery were included. CRP was measured daily during the first 5 postoperative days. Complications, specially AL, were analyzed.
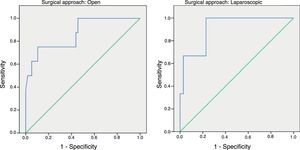
ResultsFollowing an open approach 32 patients (45.7%) presented complications, 15 (18.7%) in the laparoscopic group and 12 (29.4%) in the converted group (P=0.153). Following open surgery 9 patients experienced AL, 5 were detected in the laparoscopic group and none in those converted (P=0.153). There were significant differences in CRP values between the 3 groups (P=0.03). ROC Curves showed AUC for the open and laparoscopic approach of 0.731 and 0.760 respectively. On day 4 the AUC was 0.867 for the open group and 0.914 for the laparoscopic group.
Cut-off points on day 4 were: Open: 159.2mg/L; sensitivity 75%, specificity 89% and NPP 96% (P<0.001). Following laparoscopic surgery the cut-off point was 67.3%; sensitivity 100%, specificity 89.5% and NPP 100% (P=0.016).
ConclusionCRP on day 4 is useful to diagnose AL. Different cut-off values should be taken into account depending on the approach used.
La fuga anastomótica (FA) es una complicación seria en cirugía colorrectal, dado que conlleva un aumento de la morbimortalidad. El objetivo de este estudio prospectivo no aleatorizado es determinar si la proteína C reactiva (PCR) es útil como predictor de FA en pacientes intervenidos por vía laparoscópica versus cirugía abierta.
MétodosSe incluyeron 168 pacientes intervenidos de manera electiva por enfermedad colorrectal. La PCR fue medida diariamente en los 5 primeros días del postoperatorio. Se analizaron las complicaciones y, especialmente, la FA.
ResultadosPresentaron complicaciones 32 (45,7%) pacientes del abordaje abierto, 15 (18,7%) del laparoscópico y 12 (29,4%) en el grupo de convertidos a cirugía abierta (p=0,002). Desarrollaron FA 9 pacientes del abordaje abierto, 5 de los del laparoscópico y ninguno del grupo que hubo que convertir (p=0,15). Hubo diferencias estadísticamente significativas de los valores de PCR entre los 3 grupos (p=0,03).
Las curvas ROC mostraron un área bajo la curva (ABC) en el día 3 para el abordaje abierto y laparoscópico de 0,731 y 0,760, respectivamente. En el día 4 obtuvimos un ABC de 0,867 en el abierto y de 0,914 en el laparoscópico.
Los puntos de corte en el día 4 fueron: en abierto 159,2mg/L; sensibilidad 75%, especificidad 89% y valor predictivo negativo (VPN) de 96% (p<0,001). En el laparoscópico fue de 67,3mg/L; sensibilidad 100%, especificidad 89,5% y VPN de 100% (p=0,016).
ConclusionesLa PCR en el cuarto día postoperatorio es útil para diagnosticar FA; se deben tener en cuenta los diferentes puntos de corte en función del abordaje quirúrgico utilizado.
The incidence of anastomotic leakage (AL) in colorectal surgery is very variable: different studies have described rates between 1% and 28%.1,2 Undoubtedly, it is a very severe complication that involves important mortality and morbidity, increased hospital stay and frequently requires a stoma with relevant functional consquences.3,4 In addition, there are important implications in oncologic patients, as there has been an observed increase in local recurrence in these patients.5
Early diagnosis is very important to be able to initiate early treatment. It has been observed that delayed start of treatment greatly increases septic complications.6
In certain instances, symptoms and complementary tests either do not adequately guide us toward the diagnosis, or can delay it. Although computed tomography is still the most extensively used diagnostic test when AL is suspected, a recent systematic review has concluded that, when analyzing its utility in colon and rectal surgery, its sensitivity is just 68%.7 C-reactive protein (CRP) is an acute-phase protein synthesized in the liver and released into the blood as a response to the stimulation of inflammatory cytokines. When the stimulus for its production ceases, CRP levels decrease rapidly, and its half-life is 19h.8 CRP has been studied as a biological marker for the early diagnosis of AL in colorectal surgery and is useful for ruling it out.9–12 This fact seems to be clear in patients with open surgery; however, in patients treated with a laparoscopic approach and, a priori, a lower immune response,13 it would be possible to expect CRP values to be lower than in open surgery. Nevertheless, there is controversy in this regard among different published articles.14
For these reasons, we proposed a study aimed at determining whether CRP predicts AL similarly in open and laparoscopic surgery.
Material and MethodsOurs is a single-center, prospective, nonrandomized study including 168 patients who underwent elective surgery for colorectal disease with primary anastomosis, using open or laparoscopic approaches. The study was approved by the Ethics Committee of the hospital, and all patients gave their written informed consent.
The exclusion criteria were: age under 18, urgent surgery, active infection at the time of surgery, creation of a protective stoma, or no signed informed consent. Patients with stomas were excluded to avoid selection bias, because, although protective stomas do not prevent AL, they do reduce symptoms.
Patients were divided into 3 groups: open surgery, laparoscopic surgery and laparoscopy converted to open surgery. Conversions were not included in the open surgery group to avoid possible selection bias.
In addition to the demographic data, other risk factors were collected, such as age, sex, body mass index (BMI), existence of comorbidities: arterial hypertension; diabetes mellitus; chronic kidney failure; chronic obstructive pulmonary disease; acute myocardial infarction or cardiopathy and cirrhosis; toxic habits (alcohol and tobacco); the American Society of Anesthesia (ASA) scale; use of corticosteroids, immunosuppressants or anticoagulants; need for perioperative transfusion; neoadjuvant therapy; indication for surgery; surgical procedure performed; intention of the surgery; existence of metastasis upon diagnosis; surgical technique; surgical approach; type of anastomosis; complications during surgery; operating time; use of drain tubes; distance to anal margin; and tumor stage.
AL was defined as the existence of peritonitis during re-operation, discharge of fecaloid content through a drain tube or surgical wound, contrast extravasation seen on a barium enema test, or presence of air or a collection in an area close to the anastomosis, as detected by computed tomography scan. Minor AL (Clavien-Dindo I-II) was defined as leaks that did not require any therapeutic intervention, while major leaks (Clavien-Dindo III-V) required percutaneous drainage or re-operation.10
The patients were evaluated daily after surgery, checking temperature, abdominal exploration, bowel movements and the appearance of drainage, if any. Blood work was done at 8:00 am, including leukocytes, neutrophils, CRP, hemoglobin and hematocrit. CRP was measured the first 5 days of the immediate postoperative period by means of colorimetric immunoassay (Dimension RXL, Siemens).
All patients were followed-up for 30 days post-op.
Statistical AnalysisThe data obtained were analyzed using SPSS version 20.0.0 software (IBM Corporation, Chicago, IL, USA). A descriptive analysis was done of the patients’ characteristics using measures of central tendency and dispersion (mean and standard deviation [SD]) for continuous variables and frequency distribution for qualitative variables. The incidence was calculated with its respective 95% confidence interval (95% CI). A bivariate analysis was used to detect differences between patients using the chi-squared or Fisher's test for independent variables. The Mann–Whitney U test was used for independent quantitative variables with a comparison of 2 groups and the Kruskal–Wallis test for 3-group comparisons.
To determine the prognostic capacity of the CRP in the detection of AL, ROC curves were plotted, describing the area under the curve (AUC) with its corresponding 95% CI. The best cut-off points were determined using the Youden index, presenting the classic diagnostic test indicators: sensitivity, specificity and negative predictive value (NPV). A P<.05 was considered statistically significant.
ResultsA total of 168 patients were treated surgically, with a mean age of 63±17 years (73 women). In 71, the approach was open and in 97 laparoscopic, requiring conversion to open surgery in 17 patients.
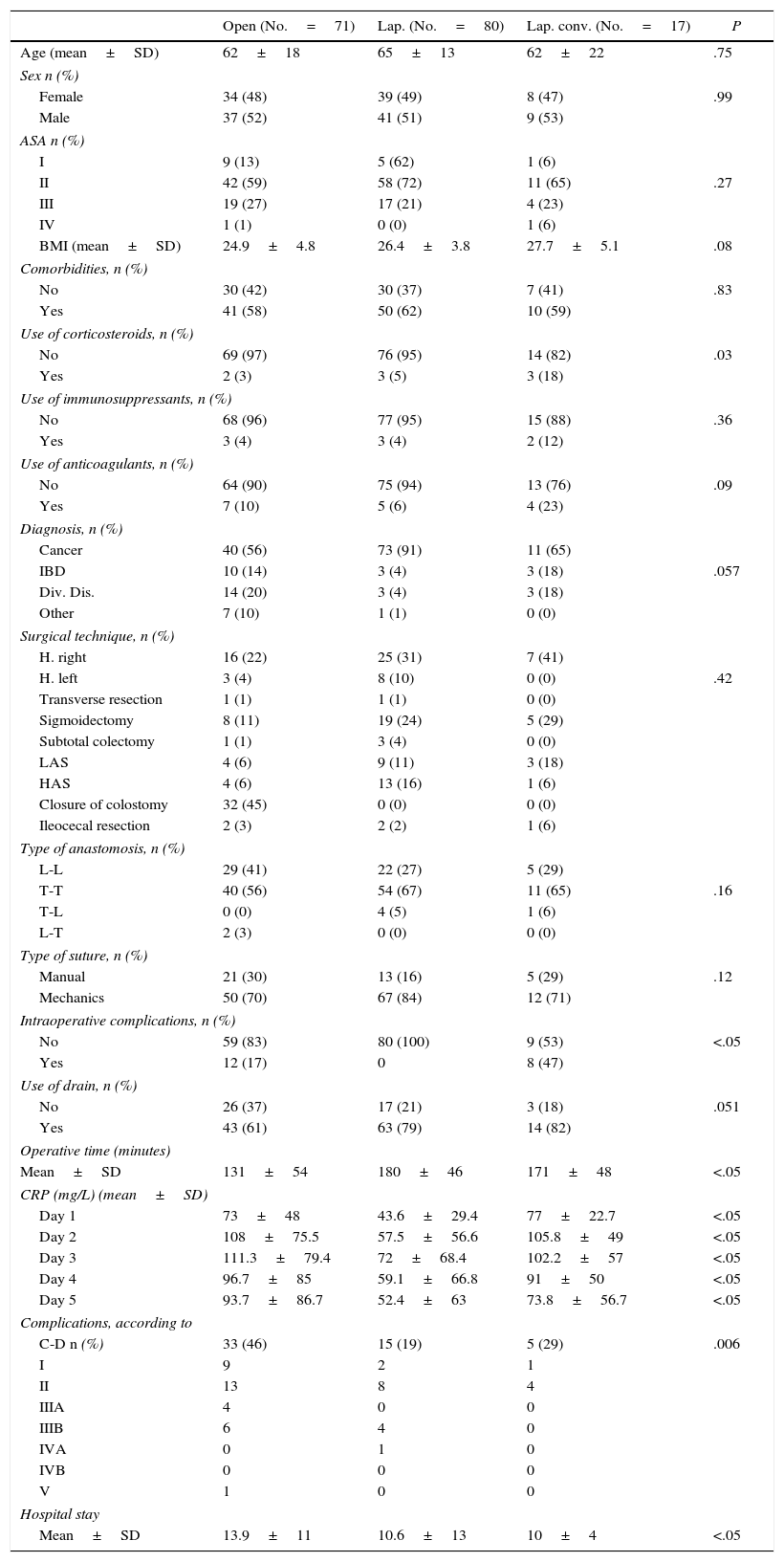
A bivariate analysis was performed to compare differences in the variables between the 3 surgical approaches. With regards to demographic data (age, sex, ASA, BMI, comorbidities, use of corticosteroids, immunosuppressants, anticoagulants and illness treated) there were no statistically significant differences among the 3 groups. The only differences found were greater surgical time in the laparoscopic group (P=.032), longer hospital stay (P=.021) and greater number of intraoperative complications in the open group, higher rate of intraoperative complications in the converted laparoscopy group (P=.006) and differences in CRP values during 5 days post-op (Table 1).
Demographic Characteristics of the Surgical Intervention and Post-op for the Treated Patients.
| Open (No.=71) | Lap. (No.=80) | Lap. conv. (No.=17) | P | |
|---|---|---|---|---|
| Age (mean±SD) | 62±18 | 65±13 | 62±22 | .75 |
| Sex n (%) | ||||
| Female | 34 (48) | 39 (49) | 8 (47) | .99 |
| Male | 37 (52) | 41 (51) | 9 (53) | |
| ASA n (%) | ||||
| I | 9 (13) | 5 (62) | 1 (6) | |
| II | 42 (59) | 58 (72) | 11 (65) | .27 |
| III | 19 (27) | 17 (21) | 4 (23) | |
| IV | 1 (1) | 0 (0) | 1 (6) | |
| BMI (mean±SD) | 24.9±4.8 | 26.4±3.8 | 27.7±5.1 | .08 |
| Comorbidities, n (%) | ||||
| No | 30 (42) | 30 (37) | 7 (41) | .83 |
| Yes | 41 (58) | 50 (62) | 10 (59) | |
| Use of corticosteroids, n (%) | ||||
| No | 69 (97) | 76 (95) | 14 (82) | .03 |
| Yes | 2 (3) | 3 (5) | 3 (18) | |
| Use of immunosuppressants, n (%) | ||||
| No | 68 (96) | 77 (95) | 15 (88) | .36 |
| Yes | 3 (4) | 3 (4) | 2 (12) | |
| Use of anticoagulants, n (%) | ||||
| No | 64 (90) | 75 (94) | 13 (76) | .09 |
| Yes | 7 (10) | 5 (6) | 4 (23) | |
| Diagnosis, n (%) | ||||
| Cancer | 40 (56) | 73 (91) | 11 (65) | |
| IBD | 10 (14) | 3 (4) | 3 (18) | .057 |
| Div. Dis. | 14 (20) | 3 (4) | 3 (18) | |
| Other | 7 (10) | 1 (1) | 0 (0) | |
| Surgical technique, n (%) | ||||
| H. right | 16 (22) | 25 (31) | 7 (41) | |
| H. left | 3 (4) | 8 (10) | 0 (0) | .42 |
| Transverse resection | 1 (1) | 1 (1) | 0 (0) | |
| Sigmoidectomy | 8 (11) | 19 (24) | 5 (29) | |
| Subtotal colectomy | 1 (1) | 3 (4) | 0 (0) | |
| LAS | 4 (6) | 9 (11) | 3 (18) | |
| HAS | 4 (6) | 13 (16) | 1 (6) | |
| Closure of colostomy | 32 (45) | 0 (0) | 0 (0) | |
| Ileocecal resection | 2 (3) | 2 (2) | 1 (6) | |
| Type of anastomosis, n (%) | ||||
| L-L | 29 (41) | 22 (27) | 5 (29) | |
| T-T | 40 (56) | 54 (67) | 11 (65) | .16 |
| T-L | 0 (0) | 4 (5) | 1 (6) | |
| L-T | 2 (3) | 0 (0) | 0 (0) | |
| Type of suture, n (%) | ||||
| Manual | 21 (30) | 13 (16) | 5 (29) | .12 |
| Mechanics | 50 (70) | 67 (84) | 12 (71) | |
| Intraoperative complications, n (%) | ||||
| No | 59 (83) | 80 (100) | 9 (53) | <.05 |
| Yes | 12 (17) | 0 | 8 (47) | |
| Use of drain, n (%) | ||||
| No | 26 (37) | 17 (21) | 3 (18) | .051 |
| Yes | 43 (61) | 63 (79) | 14 (82) | |
| Operative time (minutes) | ||||
| Mean±SD | 131±54 | 180±46 | 171±48 | <.05 |
| CRP (mg/L) (mean±SD) | ||||
| Day 1 | 73±48 | 43.6±29.4 | 77±22.7 | <.05 |
| Day 2 | 108±75.5 | 57.5±56.6 | 105.8±49 | <.05 |
| Day 3 | 111.3±79.4 | 72±68.4 | 102.2±57 | <.05 |
| Day 4 | 96.7±85 | 59.1±66.8 | 91±50 | <.05 |
| Day 5 | 93.7±86.7 | 52.4±63 | 73.8±56.7 | <.05 |
| Complications, according to | ||||
| C-D n (%) | 33 (46) | 15 (19) | 5 (29) | .006 |
| I | 9 | 2 | 1 | |
| II | 13 | 8 | 4 | |
| IIIA | 4 | 0 | 0 | |
| IIIB | 6 | 4 | 0 | |
| IVA | 0 | 1 | 0 | |
| IVB | 0 | 0 | 0 | |
| V | 1 | 0 | 0 | |
| Hospital stay | ||||
| Mean±SD | 13.9±11 | 10.6±13 | 10±4 | <.05 |
C-D: Clavien-Dindo; Div. Dis.: diverticular disease; IBD: inflammatory bowel disease; H: hemicolectomy; Lap. conv: laparoscopic conversion; HAS: high anterior resection; LAS: low anterior resection.
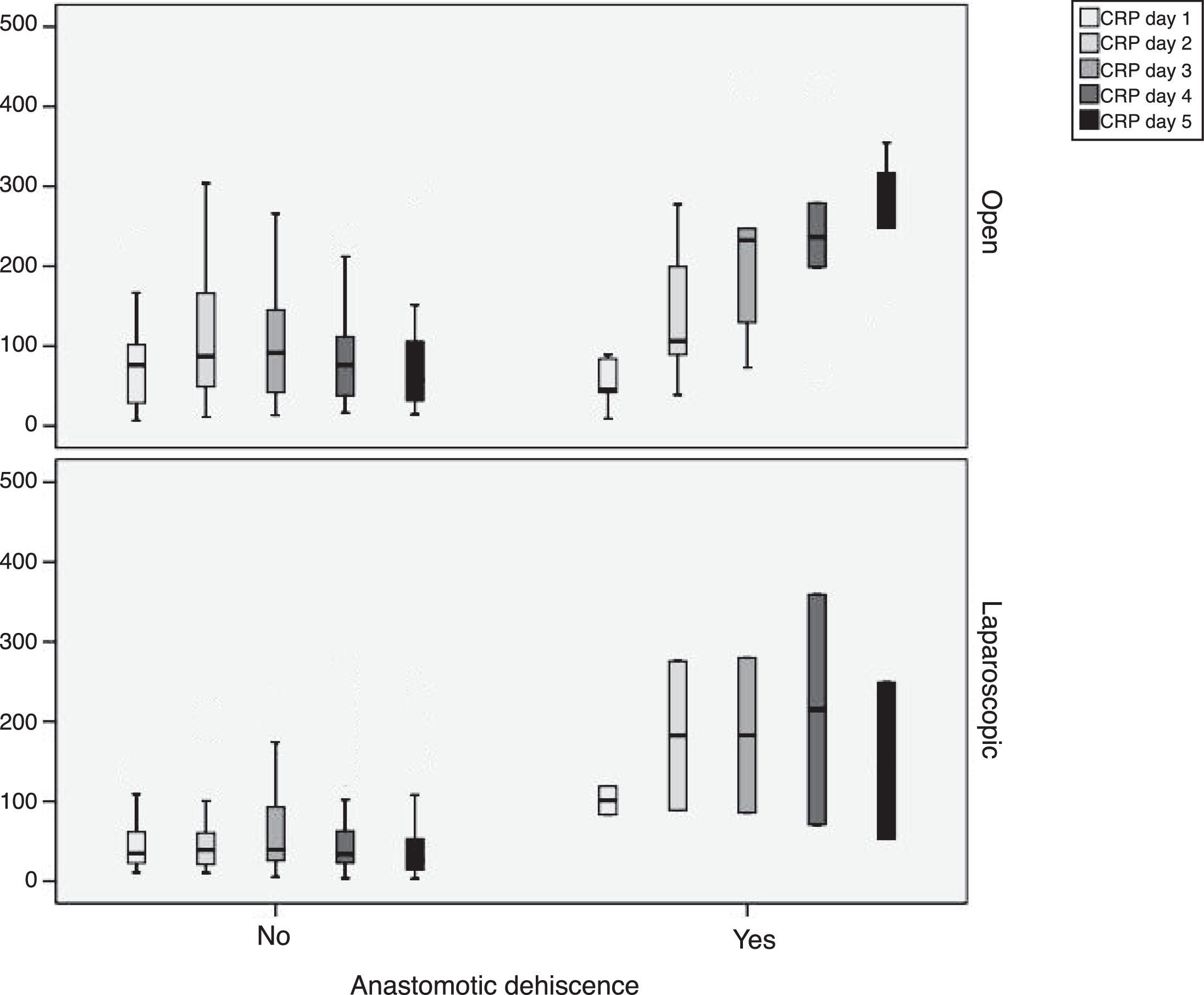
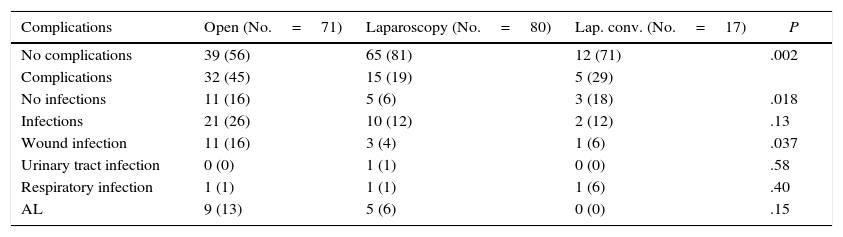
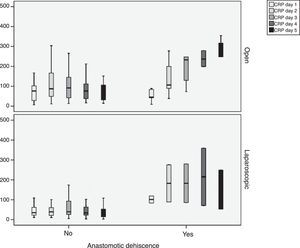
During follow-up, complications were observed in 32 (45.7%) patients treated with open surgery, 15 (18.7%) patients treated with a laparoscopic approach and 12 (29.4%) patients requiring conversion; these differences were statistically significant (P=.002). Nine patients developed AL in the open approach group, 5 in the laparoscopic group and no leaks in the conversion group. These data were not statistically significant (P=.15). In the open surgery group, there were more major leaks than in the laparoscopic group (7 versus 4, respectively), although that difference was not significant (P=.13) (Table 2). One patient died in the open surgery group (Fig. 1).
Morbidity Depending on the Approach.
| Complications | Open (No.=71) | Laparoscopy (No.=80) | Lap. conv. (No.=17) | P |
|---|---|---|---|---|
| No complications | 39 (56) | 65 (81) | 12 (71) | .002 |
| Complications | 32 (45) | 15 (19) | 5 (29) | |
| No infections | 11 (16) | 5 (6) | 3 (18) | .018 |
| Infections | 21 (26) | 10 (12) | 2 (12) | .13 |
| Wound infection | 11 (16) | 3 (4) | 1 (6) | .037 |
| Urinary tract infection | 0 (0) | 1 (1) | 0 (0) | .58 |
| Respiratory infection | 1 (1) | 1 (1) | 1 (6) | .40 |
| AL | 9 (13) | 5 (6) | 0 (0) | .15 |
AL: anastomotic leak; Lap. conv.: laparoscopic conversion.
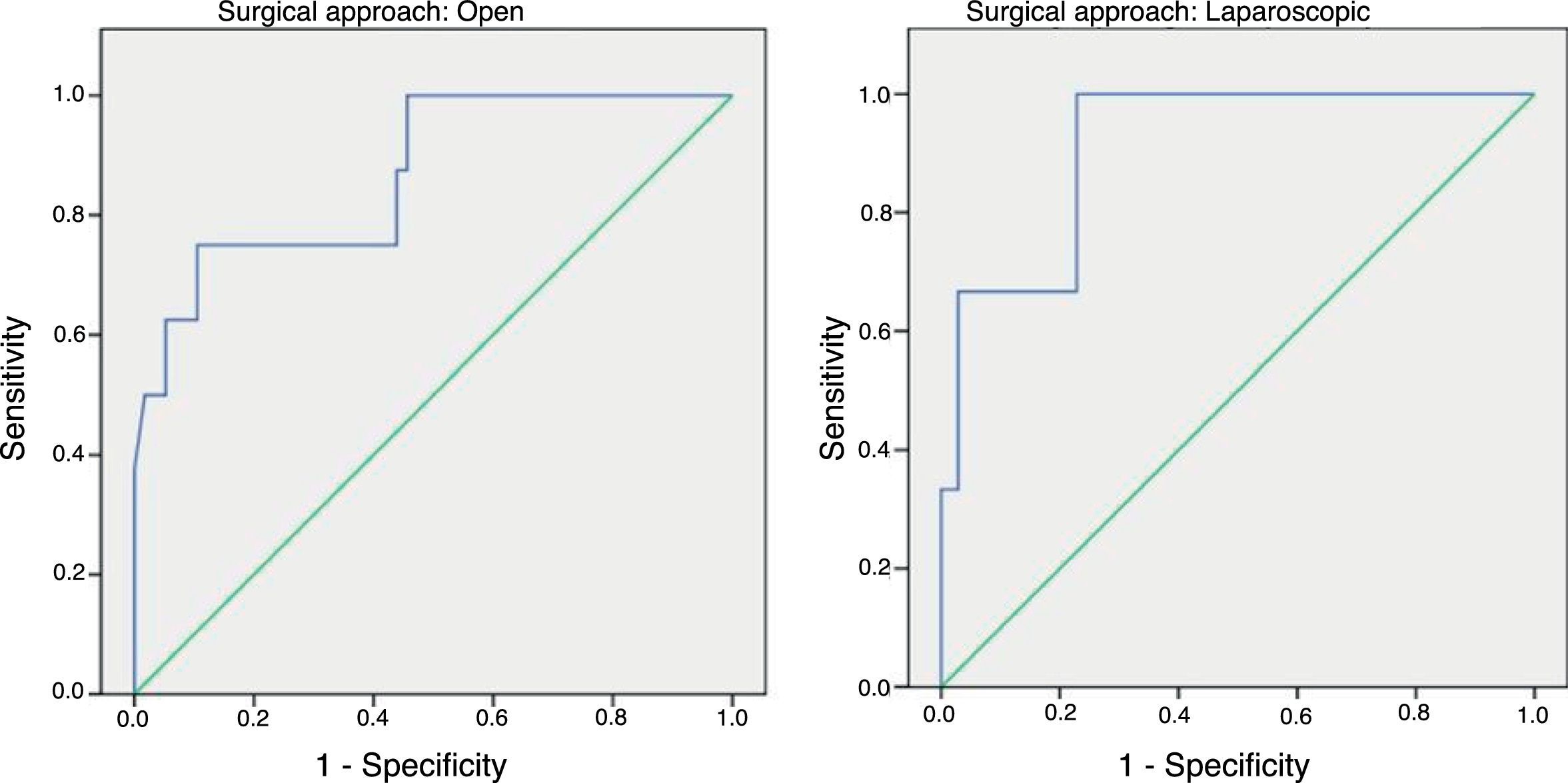
In order to establish a relationship between CRP values and the development of AL, we calculated ROC curves. Thus, we obtained an AUC on day 3 for the open and laparoscopic approaches of 0.731 and 0.760, respectively. On day 4, we obtained an AUC of 0.867 in open surgery and 0.914 with laparoscopy, which was higher than the AUC obtained on day 3 (Fig. 2).
Given that the AUC of the ROC curves was better on day 4, we calculated cut-off points for each of the approaches on this day of the postoperative period. In the case of an open approach, for a cut-off point of 159.2mg/L we obtained a sensitivity of 75% (95% CI: 38.7%–100%), a specificity of 89% (95% CI: 80.6%–98.3%) and a 96% NPV (95% CI: 90.1%–100%) (P<.001). In the case of a laparoscopic approach, a cut-off point of 67.3mg/L obtained a sensitivity of 100% (95% CI: 83.3%–100%), a specificity of 89.5% (95% CI: 66.6%–100%) and an NPV of 100% (95% CI: 99.1%–100%).
DiscussionOur results confirm that CRP is useful in the diagnosis of AL on postoperative days 3 and 4. Furthermore, our data prove that CRP levels vary according to the surgical approach performed.
Several studies have been carried out in order to determine CRP values when diagnosing infections in patients treated with colorectal surgery. In 2012, Warchkow et al.15 published a meta-analysis with 1832 patients from 6 studies, in which they assessed early discharge according to CRP value. The cut-off point that they established on the fourth day of the postoperative period was 135mg/L, with an NPV of 89%, 68% sensitivity, 83% specificity and an AUC of 0.810. Only 2 of the 6 studies were prospective and one of them had 35 patients, while the other had 133. In none of the articles is reference made to the difference in CRP related to the type of approach employed.
Regarding AL and CRP, there are several published studies that identify different cut-off points. Platt et al.16 observed that, on post-op day 3 and with a cut-off point of 190mg/L, sensitivity was 77% and specificity 80%, with an AUC of 0.84; meanwhile, on day 4 and with a cut-off point of 125mg/L, sensitivity was 77% and specificity 76%, with an AUC of 0.83. Therefore, the authors concluded that the best analytical marker for detecting/ruling out AL was CRP level on postoperative day 3. In 2010, Ortega-Deballon et al.12 published an article in which they considered CRP was a useful predictor to detect AL on day 4, since the AUC was 0.80, sensitivity 81.8%, specificity 64.4% and the NPV 95.8% for a CRP cut-off point of 125mg/L. In their prospective study comparing CRP and procalcitonin for early detection of AL, García-Granero et al.10 observed that the best marker was procalcitonin on day 5. With this marker on post-op day 5, they obtained 100% sensitivity and NPV together with a specificity of 72% and a PPV of 17% for a cut-off point of 0.31ng/mL. They also noted that CRP was useful on postoperative days 3, 4 and 5, since the AUC on these days were greater than 0.80 and the NPV were 99%, 99% and 98%, respectively. Singh et al.9 published a meta-analysis based on 7 studies with 2743 patients to see if CRP was useful as an early predictor of AL in colorectal surgery and concluded that the best day is the fourth with a cut-off point of 124mg/L, which obtained an NPV of 97%, a PPV of 21% and an AUC of 0.80.
In our study, day 4 is the best day to diagnose AL by evaluating CRP levels according to the obtained AUC, which is similar to the results of the meta-analysis. The same happens with sensitivity and NPV (around 96%); however, our CRP cut-off point differs from what was found in the meta-analysis: 124mg/L. In our study, the cut-off point was higher in the case of open surgery (159.2mg/L) and much lower in the laparoscopic approach (67.3mg/L) on day 4 post-op.
Although CRP seems to be a good marker that allows us to discharge patients more quickly as they are unlikely to develop AL, it is unclear whether this holds true when the approach is laparoscopic, since most of the studies that have been published are based on patients who have undergone open surgery.9 When a laparoscopic approach is carried out, cell immunity seems to be better preserved because there is less tissue damage and, in addition, it is less invasive. There are few studies that compare the immune response of open versus laparoscopic surgery. The articles that exist are contradictory; furthermore, the sample sizes are small.17
In 2013, Karanika et al.18 published a review of patients treated with laparoscopic colectomy and found that the immune response (expressed as secretion of CRP, interleukin 6 and other cytokines) was lower. They claimed that it was probably a consequence of the lower surgical stress that occurs with this type of approach.
There are also recent studies that assess the usefulness of CRP to diagnose infectious complications by comparing both approaches. Nason et al.19 analyzed a cohort of 169 patients in whom laparoscopic colectomy was performed and indicated that a CRP >148mg/L on post-op day 3 is suggestive of infectious complications. Ramanathan et al.14 published a study with 344 patients and concluded that, although the systemic response is higher when open surgery is performed, CRP has a similar value to predict infectious complications on days 3 and 4 post-op. Thus, on day 4 post-op and for a 140mg/L cut-off point, they obtained similar sensitivities and specificities in both approaches that were about 70%, with a 0.780 AUC for open surgery and 0.720 for laparoscopic surgery. These results differ from those found in our study when evaluating AL, since we have found differences in CRP values based on the approach used. This also occurred in the paper published by Shibata et al.,20 which included patients treated robotically, or in the Adamina et al.21 study to detect infectious complications, with an established cut-off point for CRP in the latter of 56mg/L, which is very similar to that of our research. Recently, Waterland et al.22 published an article comparing CRP values in open and laparoscopic surgery and, similar to us, they observed lower CRP values in the laparoscopic group, using a cut-off point of 91mg/L in laparoscopic surgery and 123.5mg/L in open surgery and the NPV above 97%, as in our study.
Although there are limitations in our study, such as the statistical power due to the sample size, the practical clinical relevance of our findings is not negligible.
We should conclude that CRP levels on postoperative day 4 are useful for detecting AL, but these values differ according to the approach used. Thus, in laparoscopic approaches the cut-off point is lower than in open surgery. This should undoubtedly be confirmed in future multicenter studies with a larger number of patients, as the implications in clinical practice are important
Authorship/Collaborations- •
M. Ramos Fernández was responsible for the concept and design of the present study, as well as data collection, interpretation and article design.
- •
Fernández López was responsible for data collection.
- •
F. Rivas Ruiz contributed to the analyses and interpretation of the data.
- •
F. de la Portilla de Juan contributed to the interpretation and article composition.
- •
Loinaz Segurola and J. M. Fernández Cebrián made essential contributions to the development of the study.
The authors have no conflict of interests to declare.
Please cite this article as: Ramos Fernández M, Rivas Ruiz F, Fernández López A, Loinaz Segurola C, Fernández Cebrián JM, de la Portilla de Juan F. Proteína C reactiva como predictor de fuga anastomótica en cirugía colorrectal. Comparación entre cirugía abierta y laparoscópica. Cir Esp. 2017;95:529–535.