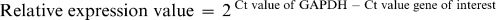Human diploid fibroblasts undergo a limited number of cellular divisions in culture and progressively reach a state of irreversible growth arrest, a process termed cellular aging. The beneficial effects of vitamin E in aging have been established, but studies to determine the mechanisms of these effects are ongoing. This study determined the molecular mechanism of γ-tocotrienol, a vitamin E homolog, in the prevention of cellular aging in human diploid fibroblasts using the expression of senescence-associated genes.
METHODS:Primary cultures of young, pre-senescent, and senescent fibroblast cells were incubated with γ-tocotrienol for 24 h. The expression levels of ELN, COL1A1, MMP1, CCND1, RB1, and IL6 genes were determined using the quantitative real-time polymerase chain reaction. Cell cycle profiles were determined using a FACSCalibur Flow Cytometer.
RESULTS:The cell cycle was arrested in the G0/G1 phase, and the percentage of cells in S phase decreased with senescence. CCND1, RB1, MMP1, and IL6 were upregulated in senescent fibroblasts. A similar upregulation was not observed in young cells. Incubation with γ-tocotrienol decreased CCND1 and RB1 expression in senescent fibroblasts, decreased cell populations in the G0/G1 phase and increased cell populations in the G2/M phase. γ-Tocotrienol treatment also upregulated ELN and COL1A1 and downregulated MMP1 and IL6 expression in young and senescent fibroblasts.
CONCLUSION:γ-Tocotrienol prevented cellular aging in human diploid fibroblasts, which was indicated by the modulation of the cell cycle profile and senescence-associated gene expression.
The free radical theory of aging suggests that the aging process involves the accumulation of oxidative damage to cells and tissues, which progressively increases morbidity and mortality (1). Human aging can be studied in vitro using normal human diploid fibroblasts (HDFs), which undergo a limited number of cellular divisions in culture and progressively reach a state of irreversible growth arrest; this process is termed replicative senescence (2).
Replicative senescence is characterized by the loss of responsiveness to mitogen-induced proliferation (3), altered growth properties, the expression of senescence-associated β-galactosidase (SA-β-gal) (4), an enlarged and flattened morphology with a concomitant increase in the nucleus and nucleoli, an increase in the number of lysosomes and Golgi, the appearance of vacuoles in the cytoplasm and endoplasmic reticulum and an increase in the number of cytoplasmic microfilaments (5). In addition to these morphological changes, the number of multinucleated senescent cells increases (6). The gradual loss of replicative potential reduces harvest cell and cell saturation densities (7). A variety of cell cycle regulation genes, including those involved in immunity and inflammation, the cytoskeleton, stress responses and metabolism, are altered during cellular senescence (8). Serial assessments of gene expression have been used to explore senescence-associated genes to gain insight into the molecular mechanisms of senescence. Several senescence-linked genes have been identified and cloned based on the changes in mRNA expression levels between young and senescent cells (9). Senescent cells accumulate in human tissues with age, which suggests that these cells contribute to organismal aging (10).
Natural vitamin E occurs in eight different forms: α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols.11 Vitamin E is the major chain-breaking antioxidant that prevents the propagation of oxidative stress, especially in biological membranes (12). α-Tocopherol modulates signal transduction and gene expression in an antioxidant and non-antioxidant manner (11). The non-antioxidant properties of α-tocopherol, including its roles in cellular signaling, gene expression, the immune response, and apoptosis, are also important (13,14).
The tocotrienol isomers have gained increasing scientific interest due to their prominent antioxidant effects and a non-antioxidant activity profile that differs from tocopherols (12) Tocotrienols are abundant in plant foods, such as rice bran and palm oil (15). Tocotrienol is more uniformly distributed in the membrane bilayer, is more efficiently recycled from its corresponding chromanoxyl radical form, and exhibits better interaction with lipid radicals compared to tocopherol. Tocotrienol exhibits better antioxidant activity than tocopherol (16). Therefore, this study determined the changes in several molecular markers of cellular aging, cell cycle profile, and senescence-associated gene expression in γ-tocotrienol-treated HDFs to elucidate the molecular mechanism involved in the prevention of cellular aging.
METHODSCell culture and the induction of senescenceThe Universiti Kebangsaan Malaysia Ethical Committee approved this research (Approval Project Code: FF-104-2007). Primary HDFs were derived from the foreskins of three 9- to 12-year-old boys after circumcision. Written consents were obtained from the parents of all subjects. The samples were aseptically collected and washed several times with 75% alcohol and phosphate-buffered saline (PBS) containing 1% antibiotic–antimycotic (PAA, Austria). The epidermis was removed, and the pure dermis was cut into small pieces and transferred to a Falcon tube containing 0.03% collagenase type I solution (Worthington Biochemical Corporation, USA). The pure dermis was digested in an incubator shaker at 37°C for 6-12 h. The cells were rinsed with PBS and cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS) (PAA, Austria) and 1% antibiotic–antimycotic at 37°C in a 5% CO2 humidified incubator. The cultured HDFs were harvested after 5–6 days using trypsinization and were culture-expanded in new T25 culture flasks (Nunc, Denmark) with an expansion degree of 1∶4. Serial passages were performed when the subcultures reached 80–90% confluency using trypsinization, and the number of population doublings (PDs) was monitored until the HDFs reached senescence. The cells were used at passage 4 (young cells, population doubling; PD<12), passage 20 (pre-senescent cells, 30
Cellular viability was assessed using the CellTiter 96* Aqueous Non-Radioactive Cell Proliferation Assay (MTS, Promega, USA). The MTS employs 3-(4,5-dimethylthiazol-2-yl)-5-carboxymethoxyphenyl) 2-(4-sulfophenyl)-2H-tetrazolium (MTS), and the electron coupling agent, phenazine methosulphate (PMS). MTS is reduced to a soluble formazan product by the dehydrogenase enzymes in metabolically active cells. The amount of colored formazan product is proportional to the number of viable cells. Briefly, 20 µL of MTS solution was added to each well and was incubated in a humidified incubator at 37°C in 5% CO2 for 2-4 h. The quantity of formazan product was determined by measuring the absorbance at 490 nm in a microtiter plate reader (VeraMax Molecular Devices, USA). The viability assay was performed to obtain the optimum dose of γ-tocotrienol (Malaysian Palm Oil Board) for subsequent experiments. Young and pre-senescent HDFs were incubated with 50 µM γ-tocotrienol, and senescent HDFs were incubated with 70 µM γ-tocotrienol for 24 h. Untreated cells were cultured in DMEM containing 10% FBS (PAA, Austria). The media for the untreated cells were changed in parallel to the treated cells. The untreated and treated cells were harvested on the same day.
Morphology analysis and senescence-associated (SA) β-galactosidase stainingThe cells were divided into six experiment groups: i) young HDFs; ii) young HDFs treated with γ-tocotrienol; iii) pre-senescent HDFs; iv) pre-senescent HDFs treated with γ-tocotrienol; v) senescent HDFs; and vi) senescent HDFs treated with γ-tocotrienol. SA-β-gal activity, the molecular marker of HDF cellular aging in vitro, was determined using a senescent cell staining kit (Sigma, USA) according to the manufacturer's instructions. Blue staining was visible after 4 h of incubation with a β-galactosidase staining solution containing 5-bromo-4-chloro-3-indolyl-β-D-galactosidase (X-gal) at 37°C. The percentage of blue cells in 100 observed cells was calculated under a light microscope.
Cell cycle analysisThe six groups of HDFs were subcultured in 10 cm2 tissue culture dishes. The cells were prepared after 24 h of incubation with γ-tocotrienol using the CycleTESTTM PLUS DNA Reagent Kit (Becton Dickinson, USA) according to the manufacturer's instructions. Cell cycle status was analyzed by flow cytometry using propidium iodide (PI) as a specific fluorescent dye probe. The PI fluorescence intensity of 10,000 cells was measured in each sample using a Becton–Dickinson FACSCalibur Flow Cytometer.
Primer designThe primers for human GAPDH, COL1A1, ELN, MMP1, IL6, RB1, and CCND1 were designed using Primer 3 software and were blasted with GenBank database sequences to obtain high-specificity primers. The efficiency and specificity of each primer set were confirmed using a standard curve (Ct value versus serial dilution of total RNA) and melting profile evaluation. The primer sequences for quantitative gene expression analysis are shown in Table 1.
Primer sequences for quantitative real-time RT-PCR.
| Gene | Accession number | Sequence (5′→3′)Sense and antisense primers | Product size, base pairs (bp) |
|---|---|---|---|
| GAPDH | NM_002046 | F: tcc ctg agc tga acg gga agR: gga gga gtg ggt gtc gct gt | 217 |
| MMP1 | NM_002421 | F: gct cct ttg gct tcc cta gaR: gca tca act ttg tgg cca at | 187 |
| IL6 | NM_000600 | F: tgg ctg aaa aag atg gat gcR: tgc agg aac tgg atc agg ac | 181 |
| CCND1 | NM_053056 | F: aga cct tcg ttg ccc tct gtR: cag tcc ggg tca cac ttg at | 181 |
| ELN | NM000501 | F: ggc ctg gag gca aac ctc ttR: cca cca act cct ggg aca cc | 189 |
| RB1 | NM_000321 | F: cag acc cag aag cca ttg aaR: ctg ggt gct cag aca gaa gg | 115 |
| COL1A1 | NM_000088 | F: agg gct cca acg aga tcg aga tcc gR: tac agg aag cag aca ggg cca acg | 222 |
Total RNA from HDFs in different groups was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. Polyacryl Carrier (Molecular Research Center) was added to each extraction to precipitate the total RNA. The extracted total RNA pellet was washed with 75% ethanol and dried prior to dissolution in RNase- and DNase-free distilled water. Total RNA was stored at -80°C immediately after extraction. A Nanodrop (Thermo Scientific, USA) was used to determine the yield and purity of the extracted RNA.
Quantitative real-time RT-PCRThe expression levels of matrix metalloproteinase 1 (MMP1), inflammation mediator (IL6), extracellular matrix (COL1A1 and ELN), and cell cycle regulatory genes (RB1 and CCND1) were quantitatively analyzed using a one-step RT-PCR technique. The expression level of each targeted gene was normalized to that of GAPDH (17). The reaction was performed using 100 ng of total RNA, a concentration of 400 nM for each primer and the iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad, Canada) according to the manufacturer's instructions. Reactions were performed in a Bio-Rad iCycler with the following reaction profile: cDNA synthesis for 30 min at 50°C; predenaturation for 2 min at 94°C; and PCR amplification for 38 cycles of 30 sec at 94°C, 30 sec at 61°C. A melt curve analysis determined the reaction specificity. Agarose gel electrophoresis was performed to confirm the PCR products. The relative expression values of target genes were calculated using the 2−ΔΔCt method of relative quantification (18) and the following equation:
Statistical AnalysisEach experiment was performed in triplicate using at least three independent cultures with comparable results. Data are reported as the means±SD of at least three experiments. ANOVA and the Student's t-test (two-tailed) were used to compare differences between groups. p<0.05 was considered statistically significant.
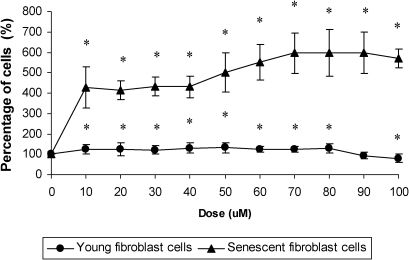
RESULTSEffect of γ-tocotrienol on cells viabilityIncubation with increasing concentrations of γ-tocotrienol for 24 h (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 µM) significantly increased the viability of young and senescent HDFs (Figure 1). γ-Tocotrienol concentrations of 50 and 70 µM exhibited the greatest percentages of viable cells in young and senescent HDFs, respectively. Therefore, subsequent experiments used 50 µM γ-tocotrienol in young and pre-senescent HDFs and 70 µM γ-tocotrienol in senescent HDFs. Higher concentrations of γ-tocotrienol reduced the viability of HDFs.
Dose response of γ-tocotrienol in young and senescent HDFs after incubation for 24 h at 37°C. Incubation with γ-tocotrienol produced a significant increase in the viability of HDFs. The percentages of viable cells were highest at γ-tocotrienol concentrations of 50 µM in young HDFs and 70 µM in senescent HDFs. However, HDF viability decreased with high concentrations of γ-tocotrienol. * Denotes p<0.05 compared to control. Data are presented as means ± SD, n = 9.
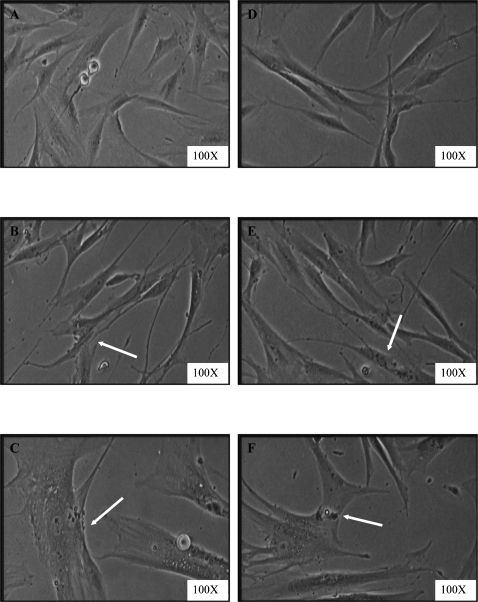
Morphological changes were observed in aging HDFs. Young HDFs displayed the normal spindle shape of fibroblast cells. However, the original fibroblastic shape was lost with senescence, and HDFs became large and flattened (Figure 2). No morphological differences were detected in the γ-tocotrienol-treated group compared to the untreated group in different passages of HDFs.
Morphological changes in HDFs in culture. Young (A), pre-senescent (B), and senescent (C) HDFs compared to γ-tocotrienol-treated HDFs (D–F). Senescent HDFs lost their original fibroblastic shape and acquiring a flattened morphology (indicated by arrow). The size of the nucleus and cell increased in treated and untreated groups. Micrographs are shown at 100X magnification.
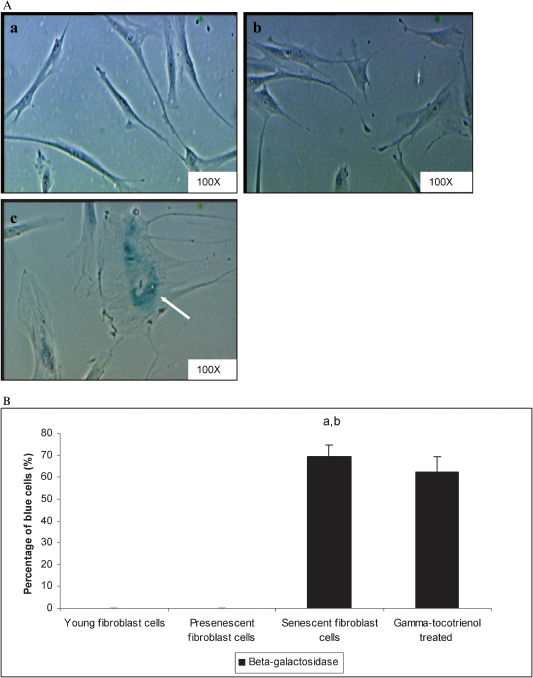
The positive blue SA-β-galactosidase stain primarily appeared in HDFs at passage 30, which suggested the presence of senescent cells (Figure 3A). Quantitative analysis revealed that the percentage of SA-β-gal-positive cells increased (p<0.05) in senescent cells compared to young and pre-senescent HDFs. The incubation of senescent cells with 70 µM γ-tocotrienol did not alter the percentage of SA-β-gal-positive cells (Figure 3B).
Senescence-associated β-galactosidase (SA β-gal) staining in young (a), pre-senescent (b), and senescent HDFs (c). Positive blue stains of SA β-gal appeared in senescent HDFs as indicated by arrows. Micrographs are shown at 100X magnification (A). Quantitative analysis of positive β-galactosidase-stained cells in HDFs during cellular aging. The percentage of cells positive for SA-β-gal staining was significantly increased in senescent cells. The incubation of senescent cells with γ-tocotrienol (70 µM for 24 h) did not produce significant changes in the percentage of SA-β-gal-positive cells. aDenotes p<0.05 compared to untreated young HDFs, bp<0.05 compared to untreated pre-senescent HDFs. Data are presented as means ± SD, n = 6.
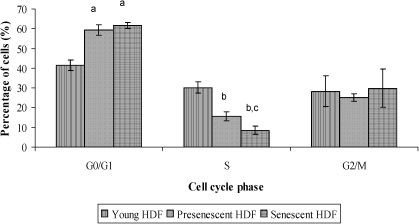
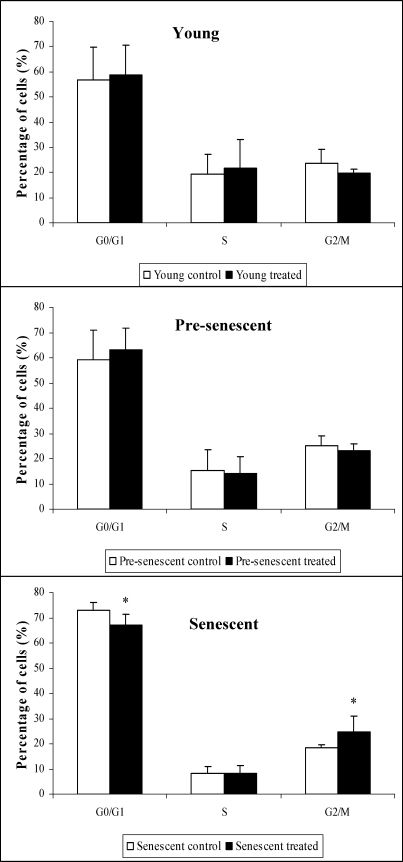
The cell populations in the S phase were significantly lower (p<0.05) in pre-senescent and senescent HDFs compared to young HDFs. The cell populations in the G0/G1 phase in pre-senescent and senescent HDFs were significantly higher (p<0.05) compared to young HDFs (Figure 4).
γ-Tocotrienol significantly decreased (p<0.05) the percentage of senescent HDFs in the G0/G1 phase. In contrast, γ-tocotrienol significantly increased (p<0.05) the percentage of senescent HDFs in the G2/M phase (Figure 5).
Effect of γ-tocotrienol on the expression of senescence-associated genesSix pairs of senescence-associated gene primers were designed. Agarose gel electrophoresis revealed that each PCR product appeared as a single band (Figure 1S). The melting curve analysis demonstrated a single and narrow peak for each PCR product (Figure 2S), which indicated that the primers and RT-PCR protocols were specific.
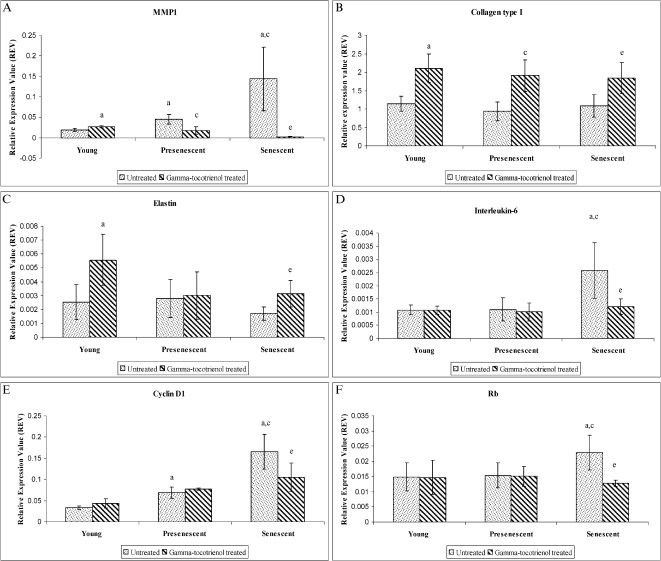
Senescent cells exhibited significant increases in MMP1, IL6, CCND1, and RB1 mRNA expression levels compared to young HDFs (p<0.05). However, γ-tocotrienol produced significant downregulations of MMP1 mRNA expression in pre-senescent and senescent HDFs compared to untreated cells (p<0.05) (Figure 6A). γ-Tocotrienol produced significant (p<0.05) upregulations of COL1A1 in young, pre-senescent, and senescent HDFs (Figure 6B). γ-Tocotrienol also significantly upregulated ELN mRNA in young and senescent HDFs (p<0.05) (Figure 6C). γ-Tocotrienol significantly downregulated IL6 (Figure 6D), CCND1 (Figure 6E) and RB1 (Figure 6F) mRNA expression in senescent HDFs (p<0.05).
Effect of γ-tocotrienol on MMP1 (A), COL1A1 (B), ELN (C), IL6 (D), CCND1 (E), and RB1 (F) mRNA expression levels in untreated and γ-tocotrienol-treated young, pre-senescent and senescent HDFs. aDenotes p<0.05 compared to control young HDFs, cp<0.05 compared to control pre-senescent HDFs, and ep<0.05 compared to control senescent HDFs. Data are presented as means ± SD, n = 6.
This study evaluated the effects of γ-tocotrienol in the modulation of cellular aging in HDFs. Our results demonstrated clear changes in cellular morphology, decreased cellular proliferation, and increased senescence-associated β-galactosidase activity in senescent HDFs. Cellular and organism morphological changes are typical features of a senescent phenotype (19). The enlargement and flattening of senescent HDFs is accompanied by changes in nuclear structure, gene expression, protein processing and metabolism (4) and an increase in the activity of senescence-associated β-galactosidase at pH 6 (6,20). The flattened cell appearance may be due to the significant upregulation of matrix metalloproteinase 1 (MMP1) mRNA and an increase in extracellular matrix degradation in aged cells. Previous study reported the overexpression of matrix metalloproteinases (MMPs) gene in senescent cells which results in the loss of proteins that maintain the ultrastructural shape. The relative overproduction of collagenase in aging cells suggests a matrix-degrading phenotype of senescent cells (21).
Our results demonstrated that γ-tocotrienol downregulated MMP1 mRNA expression in pre-senescent and senescent HDFs. This result suggested that γ-tocotrienol reduced the aging process via the inhibition of extracellular matrix degradation. Senescent HDFs exhibit lower expression levels of several extracellular matrix components, such as collagen I-1α, collagen III-1α and elastin, and increased expression levels of collagenase and stromelysin, which degrade the extracellular matrix. These data are consistent with the upregulation of MMP1 mRNA in senescent HDFs in our study. The increased expression of COL1A1 mRNA in young, pre-senescent, and senescent HDFs and the increased ELN mRNA expression levels in young and senescent HDFs demonstrated an important role for γ-tocotrienol in the maintenance of extracellular matrix architecture during various stages of cellular aging.
Senescent cells disrupt normal tissue structures and functions in complex cell culture models. For example, senescent stromal fibroblasts derange the normal organization and specialized function of mammary epithelial cells (22,23). The effects of senescent cells on mammary epithelial cells were produced by the secretion of MMPs, which is similar to the effects of senescent cells on fibrosis resolution. Osteoblasts undergo age-related cellular senescence because of the increase in oxidative stress in aged bones (24). Senescent osteoblasts alter the bone microenvironment, which contributes to the development of age-related osteoporosis (24,25). Therefore, the γ-tocotrienol-induced downregulation of MMP1 and upregulation of COL1A1 and ELN in our study suggest a medicinal role in the maintenance of normal tissue structures and functions.
Our data demonstrated an increase in cell populations in the G0/G1 phase and a decrease in S-phase cells during HDF aging. Although senescent cells display some characteristics of a late G1 arrest, these cells are actually arrested in a distinct state within a specific pathway (26). Senescent HDFs contain higher levels of oxidative DNA lesions compared to early passage cells, which suggests that oxidative damage triggers the onset of cell cycle checkpoints in senescent cells (27). The presence of damaged DNA activates checkpoint pathways, which inhibit the progression of cells through the G1 and G2 phases and induce a delay in cell cycle progression through the S phase. These checkpoints allow sufficient time to repair the damaged DNA prior to the resumption of cell cycle progression (28). Therefore, an increase in the number of cells in the G2/M phase in γ-tocotrienol treated senescent HDFs suggested that γ-tocotrienol decreased the amount of damaged DNA in aged cells and modulated the expression of cell cycle regulatory genes. Our previous results showed that a tocotrienol-rich fraction protected against DNA damage in senescent HDFs via the prevention of oxidative stress-induced DNA damage or the enhancement of the DNA repair mechanism (29).
The reduction in S-phase cells with senescence suggests a slowing of cellular proliferation when entering replicative senescence. The increase in the expression of the cell cycle regulatory genes RB1 and CCND1 that was observed in this study may explain this reduction. RB1 mRNA is intimately involved in cellular aging in senescent HDFs. Senescent cells do not phosphorylate the Rb protein, which leads to inactivation of Rb and cellular progression into the S phase (30). The impairment of this mechanism in senescent cells increases the expression of Rb and retains Rb in a hypophosphorylated inhibitory form. Our results demonstrated that γ-tocotrienol decreased the expression of RB1 mRNA in senescent HDFs.
Alterations in the expression or activity of gene products that are normally active in the G0/G1 to S progression likely play a pivotal role in cellular senescence (29). Our findings demonstrated an upregulation of CCND1 RNA in senescent HDFs. Senescent cells were arrested at G0/G1 phase where the expression of cyclin D1 was at the highest. Our finding was similar to a previous report which suggested that senescent cells might lack a feedback control mechanism that negatively regulates cyclin D1 expression. However, γ-tocotrienol downregulated CCND1 mRNA in senescent HDFs, reduced the cellular population in the G0/G1 phase and increased the population in the G2/M phase. These results suggest that γ-tocotrienol inhibited cell growth arrest and enhanced cellular proliferation in senescent HDFs. Our recent findings demonstrated that a tocotrienol-rich fraction (TRF) prevented cellular aging in HDFs by reversing the cell cycle arrest that is associated with cellular senescence and promoted cell cycle progression at different stages of cellular aging (29).
Stress-like conditions and inflammation are chronic during aging (31). Cytokines are important proinflammatory mediators, and aged individuals exhibit increased serum levels of proinflammatory cytokines, such as IL-1, IL-6, TNF-α, and IL-8, compared to young individuals (32). Our data demonstrated an upregulation of IL6 in senescent HDFs compared to the young control group. However, γ-tocotrienol downregulated IL6 mRNA in senescent HDFs.
In summary, γ-tocotrienol decreased the percentage of cells in the G0/G1 phase and increased the percentage of cells in the G2/M phase. γ-Tocotrienol also modulated senescence-associated gene expression; the expression levels of MMP1, IL6, CCND1, and RB1 were downregulated, but COL1A1 and ELN expression levels were upregulated. These results support the role of γ-tocotrienol in the prevention of cellular aging in HDFs.
γ-Tocotrienol prevented the cellular aging of human diploid fibroblasts via modulation of the cell cycle profile and senescence-associated gene expression.
AUTHOR CONTRIBUTIONSMakpol S designed and approved the study, provided critical analysis and interpretation of data and was also responsible for the revision of the manuscript. Zainuddin A was responsible for the acquisition, analysis and interpretation of data and draft of the manuscript. Chua KH was responsible for the HDF primary culture optimization and significantly contributed to the acquisition of the data. Yusof YAM designed the study and was responsible for the acquisition, and interpretation of the data. Ngah WZW interpreted the data and provided critical analysis of the intellectual content of the manuscript. All the authors read and approved the final version of the manuscript.
This study was funded by the Ministry of Science, Technology and Innovation under the E-Science Fund 02.01.02. SF0027 and the Universiti Kebangsaan Malaysia grant FF-328-2009.
No potential conflict of interest was reported.