This controlled study investigated metabolic changes in non-vaccinated individuals with Long-COVID-19, along with their connection to the severity of the disease. The study involved 88 patients who experienced varying levels of initial disease severity (mild, moderate, and severe), and a control group of 29 healthy individuals. Metabolic risk markers from fasting blood samples were analyzed, and data regarding disease severity indicators were collected. Findings indicated significant metabolic shifts in severe Long-COVID-19 cases, mainly a marked drop in HDL-C levels and a doubled increase in ferritin levels and insulin resistance compared to the mild cases and controls. HDL-C and ferritin were identified as the leading factors predicted by disease severity. In conclusion, the decline in HDL-C levels and rise in ferritin levels seen in Long-COVID-19 individuals, largely influenced by the severity of the initial infection, could potentially play a role in the persistence and progression of Long-COVID-19. Hence, these markers could be considered as possible therapeutic targets, and help shape preventive strategies to reduce the long-term impacts of the disease.
Long-lasting effects of COVID-19, known as Long-COVID-19 (LC), have been increasingly recognized. Long-COVID-19 syndrome refers to the persistence of symptoms beyond three weeks after the initial diagnosis of COVID-19. The incidence of Long-COVID-19 is higher in cases where the disease is more severe.26 The severity of COVID-19 is associated with various multisystem pathologies affecting cardiovascular, gastrointestinal, nervous, and immunologic systems, which are distinct from previous SARS strains.42,12
The widespread binding of the SARS-CoV-2 virus to the ACE2 receptor in different tissues and organs contributes to the systemic spread of infection.36 The persistence of inflammation, often characterized by a cytokine storm,42,19 can lead to organ damage and disease progression, potentially causing long-term complications.30 Reports have emerged linking COVID-19 infection to cerebrovascular disease and recurrent vascular events,4,18 indicating the prolonged effects and Long-COVID-19 symptoms of the disease may be responsible for these cardiovascular issues.38,5
Notably, cardiovascular complications such as acute myocardial infarction and stroke can be triggered by several acute respiratory viral infections, including the influenza virus. However, patients with COVID-19 are more likely to develop serious complications that endure for an extended period. This is reflected in studies that showed that the incidence of myocardial infarction increased 5 times within the first 14 days post-COVID-19 acute infection,25,35 and that COVID-19 patients are 7-fold more likely to have a stroke than patients with influenza. In addition, the risk for both acute myocardial infarction and stroke remains high for up to 1 year after infection. COVID-19 disease was associated with increased incidence and prevalence of Acute Coronary Syndrome (ACS) events. Reports showed that ACS cases increased from an incidence of 1.01 % in 2019 to 3.31 % in 2020. Adverse cardiovascular events in COVID-19 disease are reportedly attributed to factors including the marked inflammatory response, blood clotting, endothelial dysfunction and viral entry.14
Although metabolic alterations are acknowledged in acute COVID-19 disease, their role in Long-COVID-19 as risk factors remains underexplored in the existing literature. This prompted this study to investigate metabolic changes in Long-COVID-19 patients, particularly in association with disease severity, duration, prognostic and predictive value. The authors specifically focused on established acute phase measures that constitute major metabolic risk indicators, including cholesterol and glycemic profiles, as well as factors associated with oxidative stress. It is crucial to emphasize that these measures ought not to be exclusively perceived as markers indicative of the acute phase; rather, their inherent functional roles and predictive capacities in terms of disease progression should be acknowledged.
This study aims to investigate the metabolic changes in a specific group of Long-COVID-19 individuals who were affected by the original alpha strain and were unvaccinated. By examining mild/moderate and severe cases, the authors’ aim is to determine the biochemical changes occurring after recovery from the initial acute infection during the Long-COVID-19 phase and their link to disease severity. Understanding these metabolic alterations in Long-COVID-19 patients can provide insights into the potential mechanisms behind the disease's long-term effects and identify metabolic markers that could be targeted for therapeutic interventions and preventive strategies to mitigate the long-term impact of Long-COVID-19.
Materials and methodsThis prospective study received ethical approval from the Ethics Committee of Sultan Qaboos University, Muscat, Oman (SAU-EC /360/2020, MREC # 2241) on November 22, 2020.
Population and sample collectionBetween January 2021 and April 2021, blood samples were collected from 88 unvaccinated Long-COVID-19 patients who had previously completed the quarantine period. Additionally, 29 healthy individuals who had neither been affected by coronavirus nor vaccinated were included as controls. Participants with a history of diabetes, cardiovascular disease, or lipid-lowering medications were excluded from the study. A detailed electronic questionnaire was administered to gather information on persistent symptoms, illness severity during the acute infection, medications, and medical history. Prior to sample collection, Long-COVID-19 patients had to meet the de-isolation criteria set by the Oman Ministry of Health, which included being asymptomatic for 72 h without medications and completing a 14-day period from the onset of symptoms.2 All participants fasted for approximately 7‒8 h before blood collection, and the collected blood samples were stored at −30 °C until analysis.
The subjects were divided into three groups based on disease severity: mild, moderate, and severe. The classification followed the specifications provided by the World Health Organization (WHO) in their Clinical Management of COVID-19: Interim Guidance, 27 May 2020.1 The criteria for each group are described in Table 1. The study included 29 controls, 55 mild/moderate cases, and 33 severe cases. The mild and moderate cases were combined into one group to avoid overlap due to the lack of objective assessment tools. The differentiation between mild/moderate and severe cases was based on factors such as respiratory rate (> 30 breaths/min), severe dyspnea, or SpO2 < 90 %. Metabolic parameters were compared between the mild/moderate and severe Long-COVID-19 groups. The parameters were also analyzed based on the duration of Long-COVID-19, which was categorized as < 4 months, 4 ‒ 6 months, and > 6 months. Commercial kits, clinical chemistry analyzers, and manual methods were used to measure the metabolic parameters according to established procedures.
Definition of different COVID-19 severities.
| Disease severity | Definition |
|---|---|
| Mild | Symptomatic patients with common COVID-19 symptoms but without evidence of severe pneumonia. |
| Moderate | Clinical signs of pneumonia without severe pneumonia or low oxygen saturation. |
| Severe | Clinical signs of severe pneumonia with respiratory distress or low oxygen saturation. |
Metabolic parameters were examined to assess differences in disease severity and duration after recovery in Long-COVID-19 patients. The parameters included total cholesterol, Low-Density Lipoprotein Cholesterol (LDL-C), High-Density Lipoprotein Cholesterol (HDL-C), Apolipoprotein B (ApoB), oxidized LDL, fasting glucose, as well as serum iron parameters (transferrin and serum iron). Measurements were performed using the Cobas 6000 analyzer (c-501, Roche Diagnostic). Insulin and ferritin levels were measured using the Cobas 6000 analyzer (e-601, Roche Diagnostic). Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), an estimate of insulin resistance, was calculated as described previously.37 Glycated Hemoglobin (HbA1c) was measured using the Cobas Integra (400 plus, Roche Diagnostic) autoanalyzer. Oxidized LDL was measured using an Oxidized LDL Assay Kit (MDA-LDL, Human) ELISA Kit (ab242302).
Analysis and statistical testsStatistical Package for the Social Sciences (SPSS), version 28, was used for data analysis. Differences between the mild/moderate and severe groups were analyzed using multivariate analysis, adjusting for Long-COVID-19 duration, age, and gender. Eta squared was calculated as a measure of effect size to determine the strength of the association. Spearman correlation analysis was performed to investigate associations between non-parametric continuous variables.
ResultsSurvey results and persistence of symptomsThe study included males and females from 88 Long-COVID-19 patients with an age range (20‒66 years), as well as a control group with an age range (22‒54 years). The cases that participated in the study are detailed in Table 2.
Table 3 presents the percentage of patients enduring persistent symptoms in the Long-COVID-19 population after recovery. From the data collected, it can be observed that a range of symptoms persisted in patients with Long-COVID-19 after recovery. The percentages indicate the occurrence of each symptom in the mild/moderate and severe cases. Notable findings included loss of taste and smell which surprisingly showed a markedly higher percentage in the mild/moderate group compared to the severe group. A higher percentage of Long-COVID-19 patients from the severe group experienced mood swings and weight loss compared to the mild/moderate group, Fatigue was substantial in both groups, while other symptoms persisted to varying degrees in mild/moderate and severe cases.
Metabolic symptoms in long-COVID-19 patient groupsAs shown in Table 4, there was a significant increase in insulin and Insulin Resistance (HOMA-IR) in the severe group compared with controls and the mild/moderate groups. In addition, HbA1c levels showed an increased trend in the severe cases compared to the mild/moderate and control groups. In addition, HDL-C levels were found to be significantly lower in the severe Long-COVID-19 group compared to the mild/moderate and control groups. There was also a significant difference in HDL-C levels between the mild/moderate and control groups.
Multivariate comparison between severe, mild/moderate, and controls for the different metabolic parameters in LC patients.
| Metabolic Parameters | Control (n = 29) | Mild/Moderate (n = 55) | Severe (n = 33) |
|---|---|---|---|
| Sugar Profile | |||
| Fasting blood glucose (FBG) (mmoL/L) | 5.1 ± 0.5 | 5.5 ± 0.1 | 5.9 ± 0.9 |
| Insulin (mIU/L) | 14.2 ± 12.8 | 14.7 ± 8.5 | 24.2 ± 15.9a,d |
| HOMA-IR | 3.4 ± 3.5 | 3.8 ± 2.8 | 6.5 ± 4.5a,d |
| HbA1c % | 5.2 ± 0.39 | 5.4 ± 0.6 | 5.7 ± 0.8 |
| Lipid Profile | |||
| Total Cholesterol (TC) (mmoL/L) | 5.2 ± 1.0 | 5.1 ± 1.1 | 5.3 ± 1.1 |
| ApoB (mmoL/L) | 0.9 ± 0.3 | 0.96 ± 0.28 | 1.1 ± 0.3 |
| HDL-C (mmoL/L) | 1.5 ± 0.5 | 1.3 ± 0.4a | 1.1 ± 0.2b,c |
| LDL-C (mmoL/L) | 3.1 ± 0.9 | 3.0 ± 1.0 | 3.4 ± 1.0 |
| Oxidized LDL (μg/dL) | 1.7 ± 0.28 | 1.5 ± 0.14 | 1.9 ± 0.3 |
| Iron Profile | |||
| Ferritin (μg/L) | 108.0 ± 150.5 | 110 ± 117.6 | 207.3 ± 156.0b |
| Transferrin (g/L) | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.5 |
| Iron (μmoL/L) | 14.9 ± 6.6 | 15.8 ± 6.0 | 15.1 ± 5.3 |
The data showed a significant twofold increase in ferritin levels in the severe cases compared with the mild/moderate and control groups. On the other hand, iron levels showed a decreasing trend in the severe group compared with the mild/moderate Long-COVID-19 cases. All the other acute phase metabolic parameters measured in this study described in Table 4 did not show significant differences between mild/moderate, severe, and control groups.
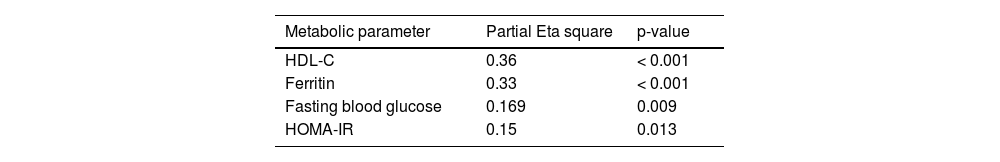
Correlation between disease severity and metabolic parameters in long-COVID-19The effect of severity on all measured metabolic parameters Long-COVID-19 was examined by multivariate analysis that was adjusted for gender and age represented by partial Eta square. The results in Table 5 show that level of severity determines 36 % of the variation in HDL-C and 33 % in ferritin levels.
Spearman correlation of HDL-C with different metabolic parametersThe correlation of HDL-C, with severity-linked metabolic parameters, showed that the strongest correlation was with ferritin (r = −0.32, p = 0.003), followed by HOMA-IR (r = −0.226, p = 0.012). Notably, no significant correlations were found between HDL-C and other iron parameters.
Association between Long-COVID-19 duration and metabolic parameters in Long-COVID-19 overall, the study found that patients with Long-COVID-19 experienced persistent symptoms and metabolic changes. The severity of the disease was associated with significant alterations in metabolic parameters including insulin, HOMA-IR, HDL-C, and ferritin levels. The duration of Long-COVID-19 also had an impact on HDL-C, ferritin, insulin, and HOMA-IR levels which are mostly apparent in severe cases.
Table 6 shows the effect of Long-COVID-19 time duration on various metabolic parameters. In the mild/moderate Long-COVID-19 cases, there were no significant differences in metabolic parameters in the different time durations after recovery as shown in Table 6 (A). Remarkably, the results in Table 6 (B) showed that in severe Long-COVID-19 cases, HDL-C levels were significantly lower up to four months after infection, and only slightly increased during the 6-month duration, but remained lower than the mild/moderate cases and controls. Ferritin, HOMA-IR and HbA1c, all remained higher than the mild cases and controls at 6 months.
The effect of LC duration on different metabolic parameters.
| A: Mild/moderate | |||
|---|---|---|---|
| Metabolic parameters | < 4-months Duration 1 (n = 18) | 4‒6 months Duration 2 (n = 25) | > 6-months Duration 3 (n = 17) |
| Sugar profile | |||
| Fasting blood glucose (FBG) (mmoL/L) | 5.3 ± 0.6 | 5.9 ± 1.2 | 5.4 ± 1.0 |
| Insulin (mIU/L) | 12.8 ± 6.6 | 15.1 ± 8.6 | 16.1 ± 10.1 |
| HOMA-IR | 3.0 ± 1.6 | 4.2 ± 3.1 | 4.2 ± 3.2 |
| HbA1c % | 5.2 ± 0.5 | 5.5 ± 0.7 | 5.5 ± 0.6 |
| Lipid Profile | |||
| Total Cholesterol (TC) (mmoL/L) | 5.2 ± 1.3 | 5.2 ± 1.1 | 4.8 ± 0.9 |
| ApoB (mmoL/L) | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.9 ± 0.2 |
| HDL-C (mmoL/L) | 1.4 ± 0.5 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| LDL-C (mmoL/L) | 3.0 ± 1.2 | 3.0 ± 0.8 | 3.0 ± 0.9 |
| Oxidized LDL (μg/dL) | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.2 |
| Iron Profile | |||
| Ferritin (μg/L) | 148.8 ± 136.3 | 78.3 ± 80.3 | 105.8 ± 127.7 |
| Transferrin (g/L) | 2.8 ± 0.6 | 2.8 ± 0.4 | 2.8 ± 0.4 |
| Iron (μmole/L) | 16.1 ± 6.5 | 16.0 ± 5.6 | 15.2 ± 6.1 |
| B: Severe | |||
|---|---|---|---|
| Metabolic parameters | < 4-months Duration 1 (n = 14) | 4‒6 months Duration 2 (n = 14) | > 6-months Duration 3 (n = 5) |
| Sugar profile | |||
| Fasting blood glucose (FBG) (mmoL/L) | 5.8 ± 0.6 | 6.3 ± 1.1 | 5.9 ± 0.9 |
| Insulin (mIU/L) | 26.1 ± 16.0 | 23.3 ± 13.0 | 22.5 ± 19.5 |
| HOMA-IR | 6.9 ± 4.7 | 6.6 ± 4.0 | 5.9 ± 5.0 |
| HbA1c % | 5.5 ± 0.6 | 5.7 ± 1.1 | 5.9 ± 0.7 |
| Lipid Profile | |||
| Total Cholesterol (TC) (mmoL/L) | 5.2 ± 1.1 | 5.3 ± 1.5 | 5.3 ± 0.9 |
| ApoB (mmoL/L) | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.1 ± 0.2 |
| HDL-C (mmoL/L) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2a |
| LDL-C (mmoL/L) | 3.2 ± 1.0 | 3.5 ± 1.2 | 3.5 ± 0.8 |
| Oxidized LDL (μg/dL) | 2.0 ± 0.6 | 1.8 ± 0.3 | 1.7 ± 0.7 |
| Iron Profile | |||
| Ferritin (μg/L) | 222.5 ± 183.7 | 200.5 ± 175.5 | 194.2 ± 100.2 |
| Transferrin (g/L) | 2.7 ± 0.7 | 2.8 ± 0.3 | 2.5 ± 0.4 |
| Iron (μmole/L) | 13.5 ± 4.9 | 14.6 ± 6.9 | 17.7 ± 3.4 |
Population studies continue to reveal the association of clinical symptoms with Long-COVID-19.29,20 Surprisingly, even patients with mild infections who did not require hospitalization could experience long-term medical complications.40 The investigation into the underlying causes of persistent Long-COVID-19 symptoms following infection has become a critical area of focus. The aim of these ongoing studies is to identify effective therapeutic and management strategies to address this ongoing disease. Recent reports highlighted that crushing fatigue is one of the most disabling symptoms of long COVID.8 In this study, the questionnaire-based data highlighted that fatigue and joint pain are the most prevalent symptoms in Long-COVID-19 patients. Mood swings and weight loss were more frequently reported in severe cases, while loss of taste and smell were more common in mild cases. The distribution of other symptoms was similar between mild/moderate and severe cases (Table 3).
While recognizing and understanding the symptoms of Long-COVID-19, it is highly crucial to explore the realm of serum biochemical indicators for an objective and quantitative measure of the underlying physiological changes associated with the disease. These laboratory parameters serve as invaluable tools, aiding clinicians in diagnosis, monitoring progression, and tailoring precise treatment strategies and targeted interventions.
Concerning the metabolic biochemical alterations in Long-COVID-19, the existing evidence regarding persistent metabolic changes beyond the hospitalization duration and completion of the quarantine period is both scarce and inconclusive. This is primarily due to the limited availability of reliable evidence, influenced by vaccination interference and the emergence of various strains subsequent to the initial occurrences. In this study, the authors managed to overcome this limitation by conducting a controlled approach focused exclusively on unvaccinated individuals who were most likely infected with the SARS-CoV-2 Alpha variant.
Notably, this study revealed marked biochemical shifts in markers linked to disease severity and metabolic complications in Long-COVID-19 patients. These metabolic profiles include iron, glycemic, and lipid risk markers as shown in Table 4. As for the iron profile, a significant two-fold increase in ferritin levels was found in the severe group compared to the mild/moderate and control groups, and this increase persisted for up to six months. Similar findings were reported in a recent meta-analysis, which identified high ferritin levels as one of the persistent abnormal parameters in 5 % ‒ 15 % of recovered COVID-19 patients.29 The association of ferritin with disease severity was significant and demonstrated major dynamic changes compared to transferrin and iron. Despite transferrin's predictive value in the acute phase shown previously,10 in this study exploring Long-COVID-19, transferrin, and iron levels did not exhibit significant differences related to severity and time after the initial infection. These findings align with the authors’ recent study, emphasizing a robust strong association between elevated ferritin and CRP levels consistent with the heightened COVID-19 inflammatory state in COVID-19. In contrast, transferrin levels, classified as an acute-phase negative reactant, displayed a non-significant decline when compared to the mild/moderate groups.10 Regarding glycemic metabolic risk factors, a significant increase in insulin levels and Insulin Resistance (HOMA-IR) was found in severe cases compared to mild/moderate cases and the control group (Table 4). These findings align with previous studies that reported elevated fasting insulin levels and HOMA-IR in both COVID-19 patients and recovered COVID-19 cases when compared to healthy individuals.27 The elevated insulin levels observed in this study suggest that Long-COVID-19 patients did not initially experience pancreatic damage, which is in contrast to studies showing pancreatic β-cell damage in non-survivors.35,9 HbA1C showed a mild increase that was not significant in the severe Long-COVID-19 group.
Concerning the lipid profile parameters, significantly lower levels of HDL-C were found in the severe group, while no significant differences were observed in other measured lipid markers, including LDL-C, Apo B, and oxidized LDL indicating that these parameters had normalized after recovery from acute COVID-19. The combination of markedly elevated insulin levels and decreased HDL-C levels, along with unchanged LDL-C and Apo B levels, corresponds to signs of metabolic syndrome, which is marked by insulin resistance, and often associated with cardiovascular risk. The present findings reflect the persistence of these systemic alterations with time particularly in severe Long-COVID-19 patients. Considering different time durations, Table 6 shows that the decrease in HDL-C levels, along with the significant increase in insulin, HOMA-IR, and ferritin, persisted across the time durations beyond 6 months apparent in the severe cases (Table 6B), compared to the mild/moderate cases (Table 6A) and controls. HbA1c levels demonstrated an upward trend with time in severe Long-COVID-19 cases (Table 6B), reflecting a cumulative glycemic history.
The major finding in this study was the significant association between disease severity and HDL-C, as well as ferritin, highlighting them as the main indicators linked to severity among Long-COVID-19 patients. Approximately 36 % decrease in HDL-C and 33 % increase in ferritin levels were predicted by disease severity, surpassing the association with all measured metabolic markers (Table 5). Importantly also, HDL-C levels were significantly, negatively correlated with ferritin levels (p = 0.003) and insulin resistance (p = 0.012) while no significant correlation was found with other severity indicators or other iron profile parameters.
While it is well-established that HDL plays an essential role in reverse cholesterol transport, studies have also demonstrated its significant anti-inflammatory and anti-atherogenic effects,27,3,6,16 contributing substantially to cardiovascular protection.13 Furthermore, HDL also exhibits antioxidant functions, facilitating the removal of oxidized lipids and neutralization of oxidative mediators, thus complementing the anti-inflammatory response.23,39 Additionally, with regard to the protective function of HDL, recent research has indicated that reduced levels of serum sphingosine-1-phosphate carried by HDL could potentially be a predictive factor for the severity of COVID-19 consequences.32,34,22 Therefore, exploring the role of HDL in counteracting tissue damage is crucial for understanding its significance in long-term COVID-19 and associated complications.
The link to ferritin resides in earlier studies that have indicated that dysregulation of iron storage in Long-COVID-19 might contribute to ferroptosis as a result of lipid oxidation and impaired iron homeostasis.10,15 This feature is characterized by an excessive accumulation of stored iron in the form of ferritin, and lipid oxidation products, predisposing tissue damage. The significant negative correlation between HDL-C and ferritin levels, particularly in the context of their association with disease severity, supports the notion that the marked reduction in HDL-C levels associated with disease severity, coupled with elevated ferritin levels, could potentially lead to additional tissue damage due to the compromised protective function of HDL-C.15 In this study, the higher trend seen in oxidized LDL levels in severe Long-COVID-19 patients compared to the mild/moderate cases may be partly explained by losing the protective effects of HDL in severe cases; however, normalization of LDL-C levels after an acute infection may explain why the differences in oxidized LDL did not reach significance.
Another key aspect that highlights the originality of the findings is the comparison with two recent Long-COVID-19 studies. The first study by Xu et al., conducted on a large cohort reported that dyslipidemia, characterized by increased total cholesterol, triglycerides, LDL-C, and lower HDL-C compared to controls, was associated with the severity of the acute phase of COVID-19 infection. That study's limitations included a predominantly white male participant group, the inclusion of individuals using lipid-lowering drugs, and a lack of information about the vaccination status of those with Long-COVID-19. Notably, concerning the present findings, their study did not establish a connection with iron status, which constitutes an important acute phase response element.41 The second study was conducted on unvaccinated young adults aged 18‒30 years, from the Swiss Armed Forces suggested an increased risk of developing metabolic disorders, including dyslipidemia, 180-days after SARS-CoV-2 diagnosis. The study revealed increased total cholesterol and LDL-C levels, with no differences in HDL-C between Long-COVID-19 participants and controls. Variations in virus strains, young age, predominantly male participants, and genetic predisposition may have contributed to the discrepancies regarding HDL-C variations compared to the present study.11 Several studies have indicated a connection between COVID-19 infection and dyslipidemia, emphasizing decreased levels of HDL-C and variations in other lipid markers.
However, it's important to note that these studies were confined to the acute phase of the infection and variations in these studies were attributed to differences in genetic phenotypes, underlying diseases, and medications. Unveiling long-term metabolic disturbances beyond recovery from the acute COVID-19 infection amid the growing concern about Long-COVID-19 pathological outcomes is crucial to be able to develop effective therapeutic and long-term care strategies. It's essential to emphasize that the research conducted was limited to the acute phase of the infection,33,21,28 and the differences observed in these studies were linked to genetic variations, underlying medical conditions, and medication use.43 However, it is crucial to identify metabolic disruptions that persist beyond the recovery phase of acute COVID-19 infection, especially in light of the growing concerns about Long-COVID-19 pathological outcomes and the need to create effective therapeutic and long-term care strategies.
In light of the notable metabolic changes observed in Long-COVID-19, especially with regard to HDL-C as a primary factor linked to severity, there is a critical need to further investigate the potential therapeutic effectiveness of interventions dedicated to lipid management, with a specific focus on HDL and its potential anti-inflammatory and antioxidant effects. For example, niacin, recognized for its role in lipid management, may increase HDL-C up to 25 %.17,24,7 Despite mixed results in preventing cardiovascular outcomes, the widespread use of niacin persists, highlighting its established effectiveness in enhancing HDL-C levels. Which was shown to be superior, at a recommended dose, compared to gemfibrozil, a fibrate, in increasing HDL-C, suggesting the potential applicability of niacin in addressing metabolic alterations associated with viral infections.12,31 Nonetheless, fibrates, including gemfibrozil, known for their ability to raise HDL-C levels and reduce major coronary events with minimal toxicity, may further contribute to the therapeutic strategies.36 Additionally, the emergence of Cholesteryl Ester Transfer Protein inhibitors as a novel drug class provides additional avenues for intervention in the context of altered lipid metabolism associated with viral infections and their complications.19
Importantly, however, there is growing support for targeting HDL functionality that could be more relevant to clinical outcomes. Measures associated with HDL such as ApoA-I and HDL particle concentration and sphingosine-1-p as specified earlier31 are considered more robust indicators of HDL function. Future research should explore therapies targeting HDL function and investigate whether improvements in these parameters translate into clinical benefits, the emerging HDL function hypothesis shows promise in reducing cardiovascular risk.31 This discussion emphasizes the need for personalized therapeutic approaches in managing metabolic changes during severe viral infections. Tailoring treatments to individual patient profiles, including specific dietary changes and education about metabolic health, is recommended to optimize HDL-C management.
It is important to acknowledge the limitations of this study, which include the relatively small sample size attributed to the limited time for recruitment of unvaccinated individuals amidst emerging viral strains and launching vaccination campaigns. Additionally, self-reported symptoms may have introduced bias into the findings.
ConclusionThis study provides biochemical and metabolic changes associated with Long-COVID-19 and their link to disease severity. It highlights major alterations in serum HDL-C and ferritin levels in severe COVID-19 cases. The metabolic changes observed in severe cases closely resemble the alterations found in the metabolic syndrome, which is characterized by reduced serum HDL-C levels as a major component and poses a risk to cardiovascular health.
This study highlights that HDL-C is negatively associated with ferritin, and that HDL-C and ferritin levels are the main factors predicted by disease severity compared to other metabolic or acute phase markers.
Furthermore, these metabolic shifts, along with other changes in metabolic risk factors, persist for several months post-COVID-19 in severe cases, differentiating them from mild cases and controls. This persistence suggests a potential role for these metabolic changes in exacerbating the severity and progression of Long-COVID-19.
The decline in serum HDL-C levels, which are typically protective against cardiovascular disease, coupled with an increase in ferritin, known for its role in oxidative stress, may be crucial in the progression of long COVID's adverse effects.
These findings are of significant interest and may contribute to the identification of potential markers for disease severity and progression in Long-COVID-19. Additionally, they may offer insights into therapeutic targets, aiding in the development of treatments and preventive measures for COVID-19 and its prolonged symptoms. This research is a step forward in understanding the complex effects of COVID-19 and managing its long-term impacts.
Data availability statementNot applicable.
Ethics approval and consent to participateThis prospective study was approved by the Sultan Qaboos University ethics committee (SAU-EC/360/2020, MREC # 2241) on November 22, 2020.
CRediT authorship contribution statementJamila Al-Zadjali: Data curation, Formal analysis, Methodology. Amal Al-Lawati: Data curation, Formal analysis. Nafila Al Riyami: Data curation, Formal analysis, Investigation. Koukab Al Farsi: Data curation, Formal analysis. Najwa Al Jarradi: Data curation, Formal analysis. Ammar Boudaka: Data curation, Formal analysis, Methodology. Ali Al Barhoumi: Data curation, Formal analysis, Methodology. Mohsen Al Lawati: Data curation, Formal analysis. Amani Al Khaifi: Data curation, Formal analysis. Asma Musleh: Data curation, Formal analysis. Prisca Gebrayel: Formal analysis, Investigation, Writing – review & editing. Sophie Vaulont: Formal analysis, Investigation, Writing – review & editing. Carole Peyssonnaux: Formal analysis, Investigation, Writing – review & editing. Marvin Edeas: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. Jumana Saleh: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.