To investigate the effects of hyperglycemia on left ventricular dysfunction, morphometry, myocardial infarction area, hemodynamic parameters, oxidative stress profile, and mortality rate in rats that had undergone seven days of myocardial infarction.
INTRODUCTION:Previous research has demonstrated that hyperglycemia may protect the heart against ischemic injury.
METHODS:Male Wistar rats were divided into four groups: control-sham, diabetes-sham, myocardial infarction, and diabetes + myocardial infarction. Myocardial infarction was induced 14 days after diabetes induction. Ventricular function and morphometry, as well as oxidative stress and hemodynamic parameters, were evaluated after seven days of myocardial infarction.
RESULTS:The myocardial infarction area, which was similar in the infarcted groups at the initial evaluation, was reduced in the diabetes + myocardial infarction animals (23±3%) when compared with the myocardial infarction (42±7%, p<0.001) animals at the final evaluation. The ejection fraction (22%, p = 0.003), velocity of circumferential fiber shortening (30%, p = 0.001), and left ventricular isovolumetric relaxation time (26%, p = 0.002) were increased in the diabetes + myocardial infarction group compared with the myocardial infarction group. The diabetes-sham and diabetes + myocardial infarction groups displayed increased catalase concentrations compared to the control-sham and myocardial infarction groups (diabetes-sham: 32±3; diabetes + myocardial infarction: 35±0.7; control-sham: 12±2; myocardial infarction: 16±0.1 pmol min-1 mg-1 protein). The levels of thiobarbituric acid-reactive substances were reduced in the diabetes-sham rats compared to the control-sham rats. These positive adaptations were reflected in a reduced mortality rate in the diabetes + myocardial infarction animals (18.5%) compared with the myocardial infarction animals (40.7%, p = 0.001).
CONCLUSIONS:These data suggest that short-term hyperglycemia initiates compensatory mechanisms, as demonstrated by increased catalase levels, which culminate in improvements in the ventricular response, infarcted area, and mortality rate in diabetic rats exposed to ischemic injury.
The prevalence of diabetes in developed countries is approaching epidemic proportions, and this disease has been associated with an increased risk of cardiovascular abnormalities and microvascular complications.1 In fact, clinical studies have demonstrated that diabetes, which is often associated with cardiomyopathy and cardiovascular neuropathy, is an independent risk factor for cardiovascular disease, and diabetes is associated with a two- to four-fold increased risk of coronary heart disease.2
Previous research has demonstrated that hyperglycemia in diabetic animals leads to changes in the heart that contribute to injury during and following a myocardial infarction (MI); however, the response of an uncontrolled hyperglycemic diabetic heart to ischemic injury remains controversial.3–6 Experimental studies using an ischemia/reperfusion protocol have shown that hearts from hyperglycemic rats7 and pigs8 undergoing a period of no-flow ischemia presented a reduced MI area and better recovery of ventricular function than those of normoglycemic animals, which indicates a possible cardioprotective role for hyperglycemic status.
Accordingly, exposure to a high-glucose medium or diabetes for short periods has been found to protect the heart against a variety of pathological insults, including ischemia, hypoxia, and calcium overload.8 A recent study from our group6 demonstrated that streptozotocin (STZ)-diabetic rats had a greater plasticity and cellular resistance to myocardial ischemic injury than nondiabetic rats did, as demonstrated by a reduced MI area and preserved systolic function. These results, together with the increased expression of regulatory genes involved in cardiac cellular survival and apoptosis, as well as decreased inflammatory cytokines, further suggest that hyperglycemia may play an important role after an ischemic event.
Free radical-mediated oxidative stress is intimately involved in the pathogenesis of heart failure.9 Under pathological conditions, the delicate balance between free radical production and the protective antioxidant defense system may shift to favor a relative increase in free radical-mediated oxidative stress. An increased level of oxidative stress has also been shown to accompany a hyperglycemic diabetic status;10 however, little is known regarding the oxidative stress that accompanies the experimental diabetes associated with MI. Therefore, the aim of the present study was to investigate the effects of hyperglycemia induced by STZ-induced diabetes on left ventricular dysfunction and morphometry, MI area, hemodynamic parameters, oxidative stress profile, and mortality rate in rats that had undergone seven days of MI.
MATERIALS AND METHODSExperimental DesignExperiments were performed using adult male Wistar rats (250-270 g) obtained from the animal facility at the University of São Paulo (São Paulo, Brazil). The rats were fed standard laboratory chow and water ad libitum. They were housed in collective polycarbonate cages in a temperature-controlled room (22°C) with a 12-hour dark-light cycle (light 07:00-19:00 h). The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Medical School of the University of São Paulo, and this investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985). The rats were randomly assigned to 4 groups: control sham (CS, n = 17), diabetic sham (DS, n = 17), control infarcted (CI, n = 27), and diabetes + myocardial infarction (DI, n = 27). Experimental diabetes was induced by an intravenous injection of 50 mg/kg STZ (Sigma Chemical Co., St. Louis, MO) dissolved in citrate buffer (pH 4.2). The rats were fasted overnight before the STZ injection. The control rats were injected with buffer only (10 mM citrate buffer, pH 4.5). Forty-eight hours after the STZ injection, diabetes was confirmed by the measurement of blood glucose levels above 200 mg/dL.
The MI and sham operations were performed in the respective groups 14 days after the STZ injection according to the procedures described below. The mortality rate was investigated during the final seven days of the protocol in all of the rats (n = 88) beginning after the MI surgery (to exclude the influence of anesthesia or stress from the surgical procedure). After MI, the animals, divided into 4 groups (8 animals each), underwent hemodynamic and left ventricular function evaluations, as well as oxidative stress analyses.
Myocardial InfarctionFourteen days after the STZ injection or administration of the citrate buffer, the rats in the CI and DI groups were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine, administered i.p.) and underwent MI induction by surgical occlusion of the left coronary artery, as described elsewhere.6,11 Briefly, a left thoracotomy was performed, the third intercostal space was dissected, and the heart was exposed. The left coronary artery was occluded with a single nylon (6.0) suture approximately 1 mm distal to the left atrial appendage. The chest was closed with a silk suture. The animals were maintained under ventilation until recovery. The sham rats (CS and DS) underwent the same procedures except that myocardial ischemia was not induced.
Left Ventricular Morphometry and Function EvaluationsEchocardiography was performed seven days after MI induction or sham operation by a double-blinded observer according to the guidelines of the American Society of Echocardiography. The rats were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine), and images were obtained using a 10- to 14-MHz linear transducer in a Sequoia 512 ultrasound system (Acuson Corporation, Mountain View, CA, USA) for measuring (i) morphometric parameters: left ventricular mass corrected by body weight (LV mass); left ventricular end-diastolic diameter (LVEDD); parameters of systolic function: ejection fraction (EF) and velocity of circumferential fiber shortening (VCF); (ii) parameters of diastolic function: left ventricular isovolumetric relaxation time (IVRT) and peak E deceleration time (EDT); and global function: myocardial performance index (MPI). The echocardiographic parameters were measured as described in detail elsewhere.11,12
Hemodynamic AssessmentOne day after the final echocardiographic evaluation, two catheters filled with 0.06 ml of saline were implanted into the femoral artery of each anesthetized rat (80 mg/kg ketamine and 12 mg/kg xylazine, i.p.). Twenty-four hours later, the arterial cannula was connected to a strain-gauge transducer (Blood Pressure XDCR; Kent Scientific, Torrington, CT, USA), and arterial pressure (AP) signals were recorded over a 30-min period in conscious animals using a microcomputer equipped with an analog-to-digital converter board (WinDaq, 2 kHz, DATAQ, Springfield, OH, USA). The recorded data were analyzed on a beat-to-beat basis to quantify changes in the mean AP (MAP) and heart rate (HR).11
Catalase and Thiobarbituric Acid-Reactive Substances AnalysesOne day after the hemodynamic evaluations, the animals were killed by decapitation, the hearts were rapidly removed and weighed, and the MI area of the left ventricle was identified and dissected for analysis. The remainder of the heart was placed in an ice-cold solution containing 140 mM KCl and 20 mM HEPES (pH 7.4). The hearts were homogenized using an Ultra Turrax blender and 1 g of tissue per 5 ml of a 1.15% (w/v)-KCl and 20-mM phenylmethylsulfonyl fluoride (PMSF) solution. The homogenates were then centrifuged at 600× g for 10 min at −2°C to remove nuclei and cell debris, as described elsewhere.13 The supernatants were used for catalase and thiobarbituric acid-reactive substances (TBARS) assays.
For the TBARS assay, trichloroacetic acid (10%, w/v) was added to the homogenate to precipitate proteins and to acidify the samples.14,15 This mixture was then centrifuged (1000× g, 3 min), the protein-free sample was extracted, and thiobarbituric acid (0.67%, w/v) was added to the reaction medium. The tubes were placed in a water bath (100°C) for 15 min. The absorbances were measured at 535 nm using a spectrophotometer. Commercially available malondialdehyde (MDA) was used as a standard, and the results are expressed as nanomoles per milligram of protein.
Catalase (CAT) activity was determined by measuring the decreased absorbance (240 nm) of hydrogen peroxide (H2O2). The results are expressed as picomoles of reduced H2O2 per minute per milligram of protein.15,16
Myocardial Infarction Size DeterminationsSeven days after MI, infarction size was delimited by subjective identification of the movement of the LV walls by observing the longitudinal, apical, and transversal echocardiographic views of the LV. Regions with a less-than-normal systolic thickness and portions with paradoxal movement were considered infarcted. Thus, the infarction size (%) was determined by the ratio of these regions to the total perimeter of the LV walls at the initial evaluation (one day) and seven days after MI surgery.6,11 To confirm the echocardiography measurement, the MI size was also evaluated by dissecting the fibrous scar from the remaining LV muscle. The outlines of both fragments were drawn on graph paper, and their areas were measured using the cross-point method17 with the interventricular septum considered to be part of the left ventricle.
Statistical AnalysesThe data are reported as means ± SEM. After confirming that all continuous variables were normally distributed using the Kolmogorov-Smirnov test, statistical differences between the groups were identified using two-way analysis of variance (ANOVA) or repeated-measures ANOVA followed by the Student-Newman-Keuls post-test. Pearson's correlation was used to study the association between the measured MI areas. A survival curve was constructed using the Kaplan-Meier method and the survival curves of the experimental groups were compared using the log-rank test. All of the tests were 2-sided, and the significance level was established at p<0.05. Calculations were performed with Statistical Package for Social Sciences (SPSS) software, version 12.0.
RESULTSAnimalsThe mean body weights were similar among all of the groups at the beginning of the study (∼260±5 g). As a result of diabetic induction, the DS (225±9 g) and DI (223±8 g) groups exhibited reduced body weights at the end of the experimental protocol compared with their initial values and the final values of the nondiabetic groups (CS: 274±4 and CI: 252±9 g). Furthermore, the STZ-diabetic rats (DS: 290±27 and DI: 301±37 mg/dl) had higher plasma glucose levels compared to the normoglycemic rats (CS: 90±3 and CI: 96±3 mg/dl) in all measurements.
LV Morphometry and FunctionThe echocardiographic parameters of LV morphometry and function are shown in Table 1. The evaluation performed seven days after sham or MI surgery revealed that the LV masses were similar between all the experimental animals; however, an increased LV chamber (LVEDD) was observed in the MI groups (CI and DI) compared to the noninfarcted groups (CS and DS). The MI groups (CI and DI) demonstrated systolic dysfunction, as evaluated by VCF and EF, compared to the CS and DS groups. Notably, the DI rats displayed an attenuation of systolic dysfunction, as demonstrated by increases in EF and VCF, compared with the CI rats. Similarly, diastolic function was preserved in the DI animals compared with the CI animals, as demonstrated by the IVRT echocardiographic parameter. In contrast, no differences in EDT were observed between the experimental groups. In addition, the DI group had a reduced MPI compared with the CI group, which demonstrated a lower level of global ventricular dysfunction in the diabetic rats after MI (Table 1).
Left ventricular morphometry and function one week after myocardial infarction and three weeks after STZ-induced diabetes.
| Parameters | CS | DS | CI | DI |
|---|---|---|---|---|
| Morphometric | ||||
| LV mass (g/kg) | 1.02±0.02 | 0.95±0.01 | 1.05±0.04 | 1.04±0.01 |
| LVEDD (cm) | 0.65±0.01 | 0.63±0.01 | 0.75±0.01∗† | 0.77±0.03∗† |
| Systolic Function | ||||
| EF (%) | 74±2.1 | 76±0.6 | 44±2.3∗† | 54±3.2∗†‡ |
| VCF (circ/s) (10-4) | 52±3 | 57±2 | 30±2∗† | 39±3∗†‡ |
| Diastolic Function | ||||
| IVRT (ms) | 33±2 | 30±1 | 26±1∗ | 33±1‡ |
| EDT (ms) | 1.87±0.11 | 1.95±0.10 | 1.97±0.10 | 1.83±0.09 |
| Global Function | ||||
| MPI | 0.43±0.04 | 0.44±0.04 | 0.50±0.03∗† | 0.40±0.04‡ |
Values are expressed as means ± SEM. LV mass – Left ventricular mass corrected by body weight; LVEDD – Left ventricular end-diastolic diameter; EF – Ejection fraction; VCF – Velocity of circumferential fiber shortening; IVRT – Left ventricular isovolumetric relaxation time; EDT – Peak E desacceleration time; MPI - Myocardial performance index.
The hemodynamic evaluation data are listed in Table 2. The systolic AP was reduced in all of the experimental groups compared with the CS group; however, the diabetic groups (DS and DI) displayed additional reductions compared with the CS and CI groups. Similarly, the DS and DI groups exhibited diastolic AP and MAP lower than those of the nondiabetic animals (CS and CI). HR was only reduced in the DS group compared with the other experimental groups (Table 2).
Hemodynamic parameters in control sham (CS), diabetic sham (DS), control infarcted (CI), and diabetic infarcted (DI) groups.
| CS | DS | CI | DI | p-values | |
|---|---|---|---|---|---|
| SAP (mm Hg) | 128±2 | 109±3∗ | 117±3∗† | 106±2∗‡ | <0.0001 |
| DAP (mm Hg) | 93±2 | 83±3∗ | 90±3 | 84±2∗ | 0.003 |
| MAP (mm Hg) | 110±8 | 96±3∗ | 104±2 | 98±2∗ | 0.005 |
| HR (bpm) | 364±12 | 302±8∗ | 367±6† | 366±10† | 0.002 |
Values are expressed as the means ± SEM. SAP – Systolic arterial pressure; DAP – Diastolic arterial pressure; MAP – Mean arterial pressure; HR – Heart rate.
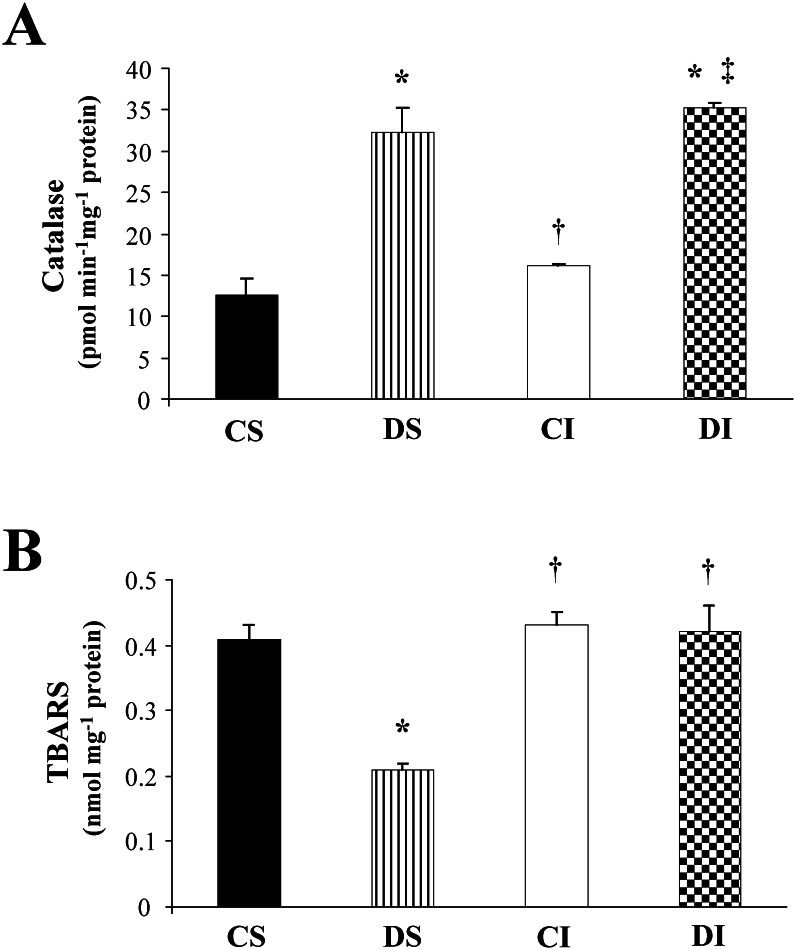
The activity of the antioxidant enzyme catalase (CAT), which catalyzes the reduction of H2O2 to H2O and O2, is shown in Figure 1A. The diabetic groups (DS and DI) displayed increased CAT activity in relation to the nondiabetic groups (CS and CI). The lipoperoxidation product, as indicated by TBARS analysis, was reduced in the DS animals compared to the CS, CI, and DI animals. Surprisingly, no difference was observed between the CI and DI rats compared to the CS rats (Figure 1B).
Oxidative profiles of cardiac homogenates in control sham (CS), diabetes sham (DS), myocardial infarction (CI), and diabetes + myocardial infarction (DI) rats (n = 8 for each group). A: Catalase activity. B: Lipid peroxidation indicated by thiobarbituric acid-reactive substances (TBARS). ∗ p<0.05 vs. CS; † p<0.05 vs. DS; ‡ p<0.05 vs. CI.
The MI area, measured by echocardiography, was similar between the infarcted groups at the beginning of the protocol (CI: 40±3 and DI: 38±3% of the LV wall). Seven days after the MI, the infarction size was reduced in the DI animals (23±3%) compared to the CI animals (42±7%). In addition to echocardiography, the MI size was also evaluated using millimeter graph paper stamps. Corroborating the echocardiographic data, the DI group showed a reduced MI area (15±6%) compared to the CI group (37±5%) and displayed a positive correlation (r = 0.9, p = 0.0001) between these two MI size measurements.
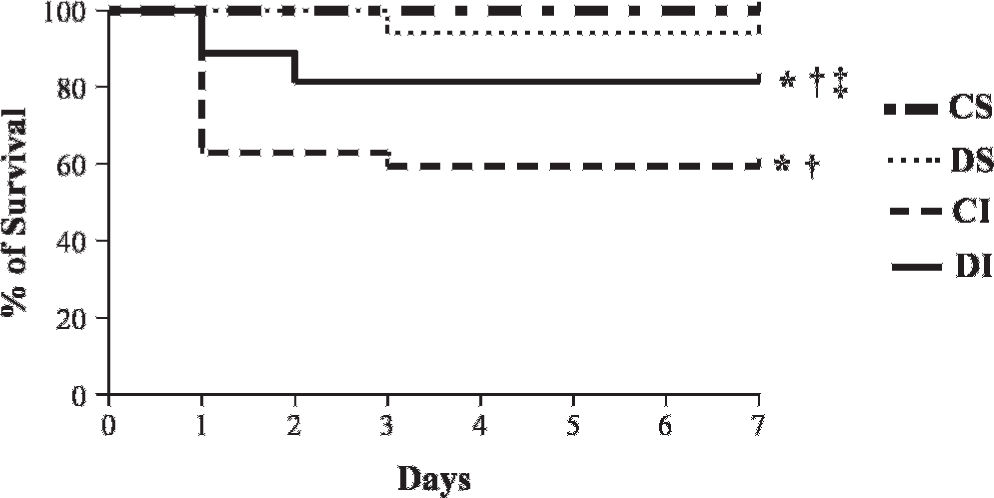
Mortality RateFigure 2 shows the total mortality rate (Kaplan-Meier survival curve) for the study groups throughout the seven-day experimental period. During the seven days after sham or MI surgery, the total mortality was similar between the CS (no deaths) and DS (1 death among 17 DS rats, 5.8%) animals. However, the mortality rate was higher (p<0.003) for the CI and DI rats compared with the CS and DS rats. Notably, the DI group (5 deaths among 27 DI rats, 18.5%) had a reduced mortality rate compared with the CI group (11 deaths among 27 rats, 40.7%; Figure 2).
DISCUSSIONThe key finding of the present investigation is that the left ventricular dysfunction induced by MI was attenuated in diabetic rats, which is an attenuation that may be associated with the reduced myocardial infarction area and the increased concentration of catalase, an important antioxidant enzyme, observed in these animals. Furthermore, these positive adaptations were reflected in a reduction in the mortality rate in the diabetic rats following MI compared with the nondiabetic rats.
Evidence has been accumulating from clinical studies with diabetic patients that an increased risk of MI and a higher rate of mortality exists in these individuals. Although it has been acknowledged that intensively controlling glucose levels in patients with diabetes reduces the risk of vascular complications, the evidence supporting the role of glycemic control in reducing cardiovascular risk is more limited. Several prospective intervention studies published in the past two years have investigated whether intensive glucose control to near normoglycemia reduces cardiovascular events and mortality in individuals with diabetes. These studies have produced conflicting results, where the use of intensive therapy to target normal glycated hemoglobin levels increased mortality and did not significantly reduce major cardiovascular events.18–20
In this sense, conflicting data have also been observed, mainly in experimental studies, and a greater resistance of hyperglycemic diabetic animals to ischemic injury has been demonstrated.6–8 Previous reports have shown that glucose supply plays a critical cardioprotective role in cardiac responses during acute ischemia. These findings suggest that a higher rate of glucose delivery to the heart may improve tolerance to ischemic stress and may lead to changes in the signaling mechanisms involved in cardioprotection.21,22 However, these protective alterations may be eliminated by preoperative treatment with insulin.23 To further investigate the mechanisms underlying the improved tolerance to ischemia in STZ-diabetic rats, we evaluated cardiac morphometry and function in the experimental groups.
Myocardial remodeling after MI is typically characterized by compensatory hypertrophy of myocytes, ventricular dilatation, and increased interstitial collagen deposition.5 In our study, LVEDD was increased in the infarcted groups compared with the noninfarcted groups; however, the CI and DI groups had similar LV mass and LVEDD values, which highlights the lack of influence of STZ-induced diabetes on cardiac remodeling after MI in this study.
In regard to cardiac function, although a reduction in SAP was observed in the DI group compared with the CI group, our data indicate that diabetes partially attenuated the ventricular dysfunction caused by myocardial ischemia (DI animals compared with CI animals). In fact, the DI animals displayed improvements in systolic (EF and VCF) and diastolic (IVRT) function indices compared to the CI animals. Along with reduced MPI, increases in these indices indicate global myocardial stress.12 These data corroborate previous findings from our group, which showed that, 15 days after MI, STZ-diabetic rats exhibited an attenuation of cardiac dysfunction, as evaluated by echocardiography, compared with normoglycemic rats.6 Notably, this attenuation was associated with improved net balances of inflammatory cytokines and regulatory genes that participate in cardiac cellular survival.6
Recently, Chu et al.8 provided evidence that diabetes induced by aloxan, which results in hyperglycemia, was protective against myocardial ischemic-reperfusion injury in pigs in the short term. The researchers observed pronounced reductions in MI area in diabetic animals, which was associated with the expression of cell survival proteins, including phosphorylated endothelial nitric oxide synthase and heat shock protein-27. Similarly, in the present investigation, the DI rats exhibited reductions in MI area compared with the CI rats, which was determined using echocardiography and was confirmed using millimeter graph paper stamps. These data are similar to previous findings from our group6 and may explain the attenuated cardiac dysfunction observed in diabetic animals.
Some studies have demonstrated that a reduction in MI size6,8,24 may be caused by the diminished number of dead myocytes in diabetic animals;25 increased capillary density; and the expression of cardioprotective proteins, including vascular endothelial growth factor, eNOS, and phospho-Akt23 and would thus be instrumental in the higher resistance of diabetic rats to ischemic injury.
In diabetes or after myocardial infarction, the augmented production of reactive oxygen species such as superoxide (O2-), hydroxyl (OH) and non-radicals capable of generating free radicals (eg, hydrogen peroxide (H2O2), leads to reductions of most antioxidant defenses9,15 and macromolecule damage. These reactive oxygen species participate in some intracellular pathways, such as the activation of various mitogen activated protein kinases, increased activation of NF-KB, disruption of calcium cycling, alteration of myofilament responsiveness to the calcium, expression of pro-fibrotic growth factors, and inflammatory cell infiltration, lading to cardiomyocyte hypertrophy, apoptosis, interstitial fibrosis, and consequently LV dysfunction.26–28
The increased TBARS levels observed in the CI group are in agreement with findings from previous studies.9 However, the DS group exhibited a reduction in TBARS levels compared to the controls. This result may be attributed to short-term exposure to diabetes or even to the increased concentration of CAT observed in the DS and DI groups. In fact, the increased CAT activity in the LV of the diabetic groups may have blocked one pathway of lipid peroxidation, which is a mechanism likely to explain the reduced TBARS levels in these animals.29 CAT activity in the heart peroxisomes of diabetic rats may be increased in response to the augmented formation of H2O2, a subproduct of the enzymatic activity of acyl-CoA oxidase that initiates the ß-oxidation of fatty acids in peroxisomes.15,30 Therefore, the reduced MI area and attenuated LV dysfunction observed in DI rats may be explained, at least in part, by the increase in CAT levels.
In conclusion, in the present investigation, we demonstrated that the MI area and left ventricular dysfunction in diabetic animals were attenuated after seven days of MI. These findings are probably caused by compensatory mechanisms associated with metabolic and molecular adjustments of cardiac tissue, particularly the increased level of catalase. Furthermore, these compensatory mechanisms culminated in a reduction in the mortality rate of DI animals compared to CI animals. These data suggest that short-term hyperglycemia may protect the heart against an ischemic insult and thus reduce the mortality rate and preserve ventricular function. It is important to emphasize that, in the present investigation, the time courses of diabetes and MI were predetermined, in contrast to what occurs in clinical situations. Therefore, it is possible that this improved cardiac function is transitory and that cardiovascular autonomic dysfunction (not evaluated in the present study) may lead to cardiovascular damage in the long term, as observed in diabetic patients following an ischemic event.
Study LimitationsSome possible limitations of the present investigation deserve comment. First, because the LV was only evaluated at the end of the protocol and necropsies were not performed on the animals that died during the seven days of the protocol, it may be difficult to predict survival outcomes (or mortality) based on these parameters. Second, in the present study, we only evaluated a small sample of anti- and pro-oxidant products; however, these evaluations may provide a general concept of the profile of oxidative stress in diabetic rats, infarcted or not.
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP- 01/00009-0; 07/57595-5) and Fundação E.J. Zerbini. B.R. received doctoral and post-doctoral scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), respectively. M.C.I and K.D.A receive financial support from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq-BPQ).