Although previous studies have been performed on cartilage explant cultures, the generalized dynamics of cartilage metabolism after extraction from the host are still poorly understood due to differences in the experimental setups across studies, which in turn prevent building a complete picture.
METHODS:In this study, we investigated the response of cartilage to the trauma sustained during extraction and determined the time needed for the cartilage to stabilize. Explants were extracted aseptically from bovine metacarpal-phalangeal joints and cultured for up to 17 days.
RESULTS:The cell viability, cell number, proteoglycan content, and collagen content of the harvested explants were analyzed at 0, 2, 10, and 17 days after explantation. A high percentage of the cartilage explants were found to be viable. The cell density initially increased significantly but stabilized after two days. The proteoglycan content decreased gradually over time, but it did not decrease to a significant level due to leakage through the distorted peripheral collagen network and into the bathing medium. The collagen content remained stable for most of the culture period until it dropped abruptly on day 17.
CONCLUSION:Overall, the tested cartilage explants were sustainable over long-term culture. They were most stable from day 2 to day 10. The degradation of the collagen on day 17 did not reach diseased levels, but it indicated the potential of the cultures to develop into degenerated cartilage. These findings have implications for the application of cartilage explants in pathophysiological fields.
Articular cartilage plays an integral role in the load-bearing ability of knee joints. In its healthy state, it provides excellent friction and lubrication to help sustain the rigorous dynamic loads that are placed on the knee joint during daily activities.1 Typical articular cartilage consists of four major constituents: water, cells, proteoglycans, and collagen networks. Both proteoglycans and collagen are responsible for the load-bearing properties of the cartilage. The primary constituent of proteoglycans is aggrecan, while the majority of the collagen fibers are formed from type-II collagen.2,3 Cartilage extracellular matrix (ECM) metabolism is regulated by chondrocytes, which constitute only 5% of the total content of cartilage. Due to its avascular and aneural characteristics, the healing ability of cartilage is limited to such an extent that partial articular cartilage defects rarely or never heal spontaneously.4 This finding has prompted extensive worldwide research on cartilage transplantation with the goal of finding a sustainable solution.
Most researchers opt to study cartilage in vitro to better understand the response of cartilage to various stimuli and to eliminate unrelated disturbances that may occur in vivo. There are various models for cartilage culture, including monolayer culture,5–8 micromass/pellet culture,9,10 scaffold culture,11,12 and explant culture.7,10, Monolayer culture is a two-dimensional culture of chondrocytes on the flat surface of a plastic dish or culture flask. Micromass/pellet and scaffold cultures are three-dimensional (3D) culture systems that provide the cells with a suitable 3D environment to secrete and build a new ECM from scratch.
Although 3D culture is clearly a better model than monolayer culture, it has been argued that chondrocytes in the newly-built matrix may act differently from those in naturally occurring ECM;8 therefore, on this basis, explant culture is advantageous. Through the direct culture of a small piece of tissue harvested directly from the host, explant culture provides a more controlled and physiologically relevant model, with both the morphological and biosynthetic characteristics of the chondrocytes remaining intact. Additionally, explant culture minimizes the complicated systemic and environmental parameters found in vivo.14,16 Thus, explant culture is generally regarded as the superior model for the study of cartilage metabolism.
Regardless of the various advantages provided by cartilage explant culture, the dynamics of cartilage metabolism after extraction from the host are still poorly understood. Admittedly, the vigorous extraction that is necessary to obtain a regularly sized cartilage explant places the adjacent cartilage under immense mechanical trauma. Thus, the primary focus of this study was to investigate the response of cartilage to the trauma sustained following explantation and to determine the time needed for stabilization. The latter consideration is particularly imperative, as the effects of the various stimuli applied to the cartilage explant can be accurately assessed only if the explant is stable. Previous research has shed some light on the dynamics of cartilage explant culture; however, our understanding of culture dynamics is incomplete and has been obstructed by differences in the anatomical location of the tissue harvests, the extraction method, the culture conditions, and the age of the tissue used in various studies.15
The aim of this study was to investigate the response of cartilage to the trauma sustained during explant extraction by observing biochemical changes (i.e., cell number, proteoglycan content, and collagen content following excision and culture of full-thickness explants with an intact subchondral bone layer). Furthermore, we aimed to determine whether chondrocytes can withstand the vigorous extraction process that is necessary for preparing an explant culture. Finally, the results of this study were compared to those of related studies.
MATERIALS AND METHODSBovine metacarpal-phalangeal joints with intact synovial membranes from skeletally mature adult cows were obtained from a local abattoir. The joints were opened aseptically and sawn laterally at the joint end to extract two large bone-cartilage samples. These bone-cartilage samples were further processed in the laminar flow hood to remove most of the bone, leaving only ∼2 mm of the bone with the cartilage on top. The sawing process was accompanied by constantly irrigating the exposed cartilage surface with sterile phosphate-buffered saline (PBS) solution at 4°C. Approximately twenty 6-mm-diameter cartilage disks were then extracted from the processed bone-cartilage samples using an in-house-built hollow punch. The thickness of the overall cartilage-bone explant was ∼3 mm. All of the explants were washed once with 70% ethanol for 30 seconds, once with sterile PBS containing antibiotics and three times with sterile PBS, with the exception of one group, which was washed with sterile PBS to assess the effect of washing with ethanol on cell viability.
All of the disks were transferred to six-well Techno-Plastic-Product (TPP) plates (Trans-Techno Enterprise, Malaysia), with four explants in each well. The explants were immobilized using 2% agar (Fisher Scientific, Malaysia) and cultured in 5 ml/well of Dulbecco's modified Eagle's medium (D5921, Sigma Aldrich, Malaysia) supplemented with 20% (v/v) fetal bovine serum (F9665, Sigma Aldrich, Malaysia), 16 mM HEPES (H0887, Sigma Aldrich, Malaysia), 1.6 mM L-glutamine (G7513, Sigma Aldrich Malaysia), 160 U/ml penicillin-160 μg/ml Streptomycin (P4333, Sigma Aldrich, Malaysia) and 0.68 mM L-ascorbate (Ducheta Biochemie, Malaysia).17
The explants were cultured at 37°C under an environment of 95% air and 5% CO2 for up to 17 days, and the medium was changed every two days.
The explants harvested on day 0 were grouped according to their washing condition. The explants in the first group were washed three times with sterile PBS, while those in second group were washed once with 70% ethanol, once with PBS containing antibiotics and three times with sterile PBS. The cell viability of the explants in each group were tested using Trypan blue exclusion.
The explants were harvested at days 0, 2, 10, and 17 of culture and washed with PBS. Full-thickness cartilages samples were extracted from the explants and individually weighed. The samples were then digested with papain (P3125, Sigma Aldrich, Malaysia) for 18-24 hours at 60°C.18 Next, the total DNA content (cell number) of the digests was analyzed using a fluorometric Hoechst 33258 assay.19 The remaining digests were stored at -80°C for future analysis and analyzed colorimetrically to measure chondroitin-6-sulfate (C6S) and hydroxyproline content. C6S content was assayed using the dimethylmethylene blue (DMB) assay.20 The digests were acid-hydrolyzed for 18 hours at 110°C before having their hydroxyproline content analyzed.21,22 All of the analytical data were normalized to the cartilage wet weight.
The results obtained were expressed as the mean ± the standard error of the mean. All of the data were pooled into four groups based on the culture time. The means of two or more different groups were compared using one-way between-groups analysis of variance (ANOVA) and Tukey's Honestly Significant Difference (HSD) test, which were calculated using Statistical Package for Social Sciences (SPSS). The mean differences between the two groups were compared using independent t-tests. The significance level was set at 0.05 (p≤0.05).
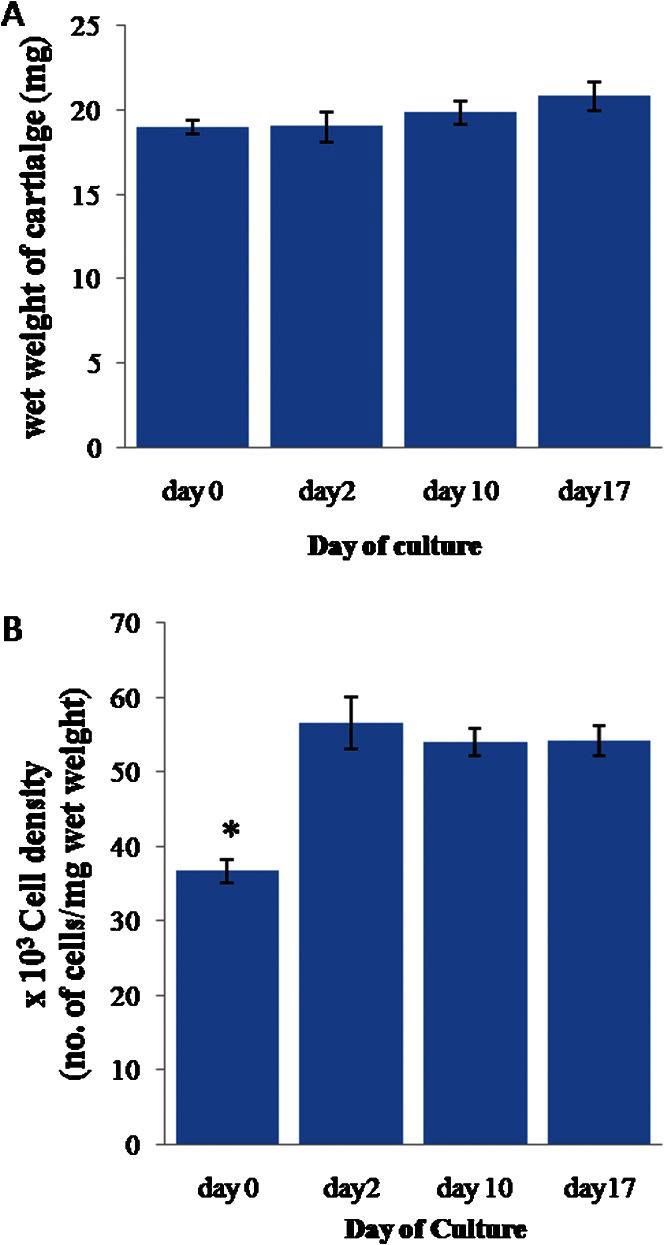
RESULTSThe effect of the washing method on the cell viability of the explants was revealed by the significantly higher (90.33±1.61%) mean cell viability of the explants in the first group (washed three times with sterile PBS; n = 8) compared to those in the second group (washed once with 70% ethanol, once with sterile PBS containing antibiotics, and three times with sterile PBS; n = 8), which had a cell viability of 83.56±1.62% (p≤0.01). The mean wet weight of the cartilage explants (Figure 1A) remained stable at 20±0.7 mg throughout the culture period.
(A) The wet weight of the full-thickness cartilage. (B) The cell density of the cartilage normalized to the cartilage wet weight. The error bars represent SEMs. n = 22, 12, 51, and 37 for day 0, 2, 10, and 17, respectively. In (B), ∗ indicates p≤0.05 compared to the results for day 2, day 10 and day 17.
The mean cell density (Figure 1B) was significantly lower at day 0 than during the remainder of the experiment (p≤0.05), with a value of 36.70±1.57 (∗103 cells/mg wet weight of cartilage). However, the cell density stabilized after day 2, with an average of 54.36±2.35 (×103 cells/mg wet weight of cartilage).
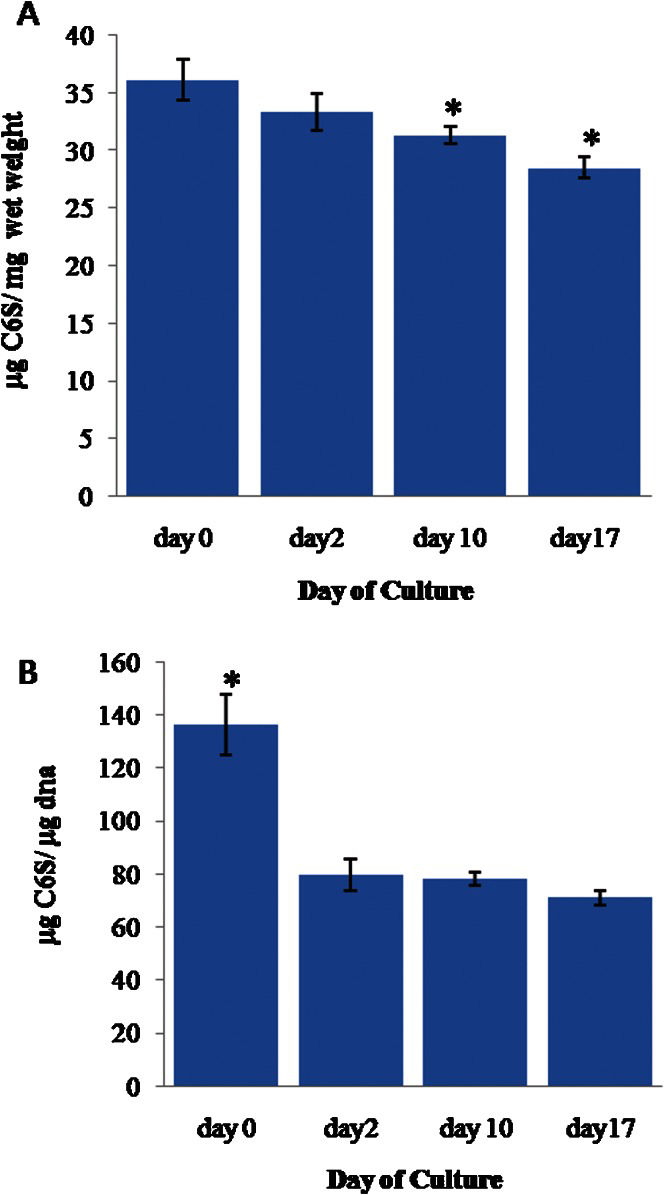
The mean C6S weight (Figure 2A), which was normalized to the wet weight of cartilage, gradually decreased between day 0 (0.036±0.002 μg C6S/mg wet weight of cartilage tissue, hereafter abbreviated as w/w) and day 17 (0.028±0.001 [w/w]) (p≤0.05). The mean C6S weight was 0.033±0.002 (w/w) on day 2; on day 10, the weight was 0.031±0.001 (w/w), which was also significantly lower than the value on day 0.
(A) The weight of chondroitin-6-sulfate (C6S) in the cartilage explants normalized to the cartilage wet weight. (B) The weight of C6S normalized to the DNA weight. The error bars represent SEMs. n = 22, 12, 51, and 37 for day 0, 2, 10, and 17, respectively. In (A), ∗ indicates p≤0.05 compared to the results for day 0. In (B), ∗ indicates p≤0.05 compared to the results for day 2, day 10 and day 17.
Similarly, the mean C6S weight normalized to the weight of DNA (Figure 2B) was found to be significantly higher (p≤0.05) on day 0 (0.128±0.009 w/w) when compared to the values at the later time points. The values were relatively stable for these groups, with an average of 0.076±0.004 (w/w).
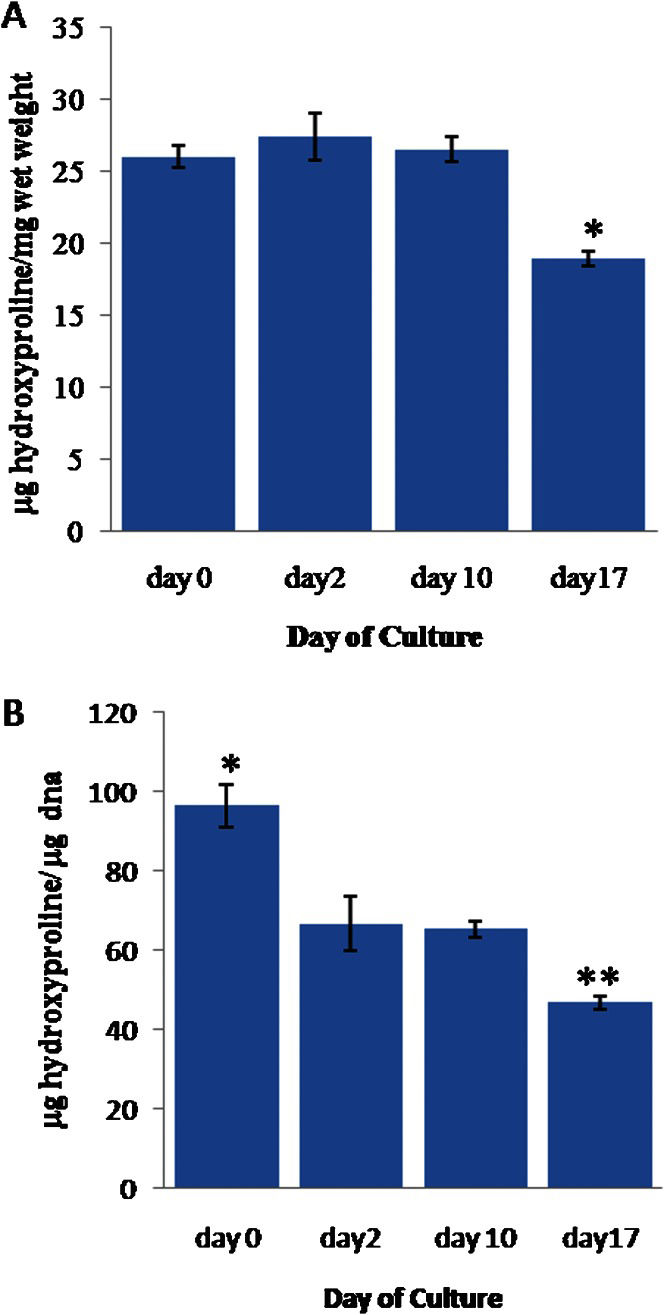
In contrast, the mean hydroxyproline weight, which was normalized to the cartilage wet weight (Figure 3A), remained stable from day 0 to day 10, with an average value of 0.026±0.001 (w/w). However, this value dropped significantly (p≤0.05) to 0.018±0.0004 (w/w) on day 17.
(A) The weight of hydroxyproline (hyp) in the cartilage explants normalized to the cartilage wet weight. (B) The weight of hyp normalized to the DNA weight. The error bars represent SEMs. n = 22, 12, 51, and 35, respectively. In (A), ∗ indicates p≤0.05 compared to the results for day 0, day 2 and day 10. In (B), ∗ indicates p≤0.05 compared to the results for day 2, day 10 and day 17. ∗∗ indicates p≤0.05 compared to the results for day 0, day 2 and day 10.
The mean hydroxyproline weight normalized to the DNA weight (Figure 3B) was significantly higher (0.096±0.005 w/w) on day 0 (p≤0.05) than at the later time points. The value stabilized at 0.063±0.003 (w/w) between days 2 and 10 but significantly decreased to 0.046±0.002 (w/w) on day 17 (p≤0.05).
DISCUSSIONA cell viability test was performed to verify that washing the explants with 70% ethanol would not have a negative effect on cell viability. We found that, although washing with 70% ethanol did significantly decrease cell viability, the viability remained at a high level of ∼80%. The suitability of this treatment was further demonstrated by the fact that the chondrocytes responded with an increase in cell density after two days of culture. These observations suggest that only the chondrocytes in the superficial layers were killed by the ethanol, while the others remained unaffected. This tradeoff is worthwhile, as most of the explants that were not washed with 70% ethanol became contaminated after two days of culture and had to be discarded.
The wet mass of the cartilage tissue remained stable throughout the culture period. No apparent swelling was observed visually. This observation was supported by the constant wet weight of the cartilage.
The cartilage tissues were co-cultured with the underlying subchondral bone to avoid further damage to the cartilage tissue (especially the deep-zone tissue), which could occur while cutting the cartilage tissue out of the bone layer. Furthermore, previous studies23–25 have shown that co-culturing cartilage with osteochondral explants does not significantly affect the metabolism of the cartilage, despite the potential for the release of chemical factors, such as cytokines and/or free radicals.
The fluorometric assay measured the DNA content of only the live cells, as most of the DNA from the dead cells was lost during DNA extraction and discarded along with the cell debris pellet.26 We found that the cell density was the lowest on day 0, with a value that was ∼32% lower than the densities observed at the later time points. Due to the density of the extracellular matrix, it is unlikely that the increase in cell density after day 0 was due to cell proliferation. Instead, it is more likely that the explants studied at day 0 experienced more cell death than the explants that were given sufficient time to equilibrate. These cartilage explants were all washed once with 70% ethanol, which might have affected the chondrocytes in the superficial zone; if these cells had been given time to recover, they may have been able to survive. However, the explants on day 0 were digested immediately after the 70% ethanol wash. Therefore, those weakened cells from the superficial zone might have been unable to withstand the vigorous digestion and died, resulting in much lower cell viability. Consistent with this hypothesis, the cell density remained relatively stable after day 2, and there was no DNA accumulation throughout the culturing process, indicating that no cell outgrowth occurred.27
The chondroitin-6-sulfate (C6S) content of the explants was found to be equivalent to the proteoglycan content of healthy cartilage. It was noted that, when the C6S content was normalized to the cartilage wet weight, the value exhibited a gradual decrease from day 0. This observation is sensible if one considers that the collagen networks at the periphery of the explants were largely distorted during explant extraction. Therefore, it was inevitable that some of the proteoglycan would leak into the bathing culture medium. Although significant differences in the proteoglycan content were observed between day 0 and day 10 and between day 0 and day 17, the proteoglycan content become relatively stable after day 2, with no significant differences recorded after that time point. Additionally, when the C6S content was normalized to the DNA weight, we found that the proteoglycan content remained relatively stable after day 2. It can be argued that the high value at day 0 was due to the low cell density; furthermore, the proteoglycans bound for leakage were not given sufficient time to diffuse out of the cartilage by this assay point.
Regarding the hydroxyproline weight/cartilage wet weight (w/w), we noted that this value remained very stable from day 0 to day 10 in full-thickness cartilage. This finding agrees with previous findings that the turnover rate for collagen is the slowest of all of the cartilage components.16 However, we observed an abrupt ∼30% drop in collagen content at day 17, suggesting that the collagens were slowly degraded after 10 days of culture. Because a partial or total loss of proteoglycan always precedes collagen degradation in diseased cartilage,28–30 the cartilage studied here was not developing into diseased cartilage, as the proteoglycan content remained relatively stable through day 17. However, because the osteochondral explants were cultured for a prolonged period without any stimulation, it is possible that the “unstimulated” chondrocytes failed to maintain their normal metabolism, which led to gradual collagen degradation.16 Consistent with this hypothesis, it is generally accepted that cartilage metabolism changes according to the direct mechanical loading exerted by an individual's daily activities. It would be interesting to determine if the cartilage explants would eventually develop into diseased cartilage after a culture period of more than one month and with no stimulation.
According to a study by Hoemann,18 the glycosaminoglycan (GAG), collagen and DNA contents in 1 mg of wet mass of typical articular cartilage samples are approximately 50 μg, 150 μg and 0.2-0.6 μg, respectively. These values are comparable to the results obtained in this study, in which contents of 31 μg of GAG, 198 μg of type-II collagen (a conversion factor of 7.6 for extrapolating hydroxyproline content to type-II collagen type II31), and 0.42 μg of DNA were observed during the stable culture period. However, it should be noted that this study was performed in vitro, and the in vivo reproducibility of the biochemical content of cartilage tissue has not been verified.
The results of this study were further compared to those of previous studies by Dumont et al.14 and Brighton et al.13 The explants used in the study by Dumont et al. were similar to the explants used in this study in the sense that both were cartilage-bone explants. However, Brighton et al. cultured eight small pieces of full-thickness cartilage derived from 8-mm-diameter samples, and those eight pieces were taken as equivalent to one explant. As for the culture medium involved, Dumont et al. used serum-free DME/F12, while Brighton et al. substituted the serum for 1% insulin-transferrin-selenium G in DMEM.
The results of the biochemical analyses performed in these previous studies differ slightly from those of the current study. Part of this difference could be attributed to the various anatomical harvest positions (the shoulder joint for Dumont et al. and the patella for Brighton et al.).15 Dumont et al. reported that 4 days were required for the GAG content of their cartilage explants to stabilize at 22.5 μg/ml after dropping from an initial value of ∼31 μg/ml, while the cartilage explants in this study only needed two days to stabilize. The higher GAG value found in this study (31 μg/ml) may be due to the addition of serum.7 The average number of chondrocytes in 1 mg of cartilage after equilibration was found to be 38,300 cells/mg of cartilage, which is about 70% of the value found in this study (54,360 cells/mg of cartilage). Again, this difference could be due to the addition of serum in our study. However, the type-II collagen content was similar in both studies (∼187 μg/ml in the study by Dumont et al. and ∼198 μg/ml in this study). The only difference was that Dumont et al. found the type-II collagen content to be constant for durations of up to 21 days, while, in this study, it decreased significantly on day 17.
A comparison with the results of Brighton et al. revealed some interesting findings. Although Brighton et al. also studied the effect of electrical stimulation on cartilage explants, only the results of their control set were used for comparison. The authors found that their cartilage explants were relatively stable from day 0 of culture, and washing the explants with 70% ethanol did not appear to affect the cell number (reflected by the DNA weight). They reported a proteoglycan content normalized to DNA weight (w/w) of 65 on day 0, which was followed by an insignificant increase to 70 on day 3 and to 75 on day 10 before dropping back to 65 on day 17. In this study, we observed that the GAG content remained relatively stable, reaching an average of 76 after two days of equilibration. The results that Brighton et al. found for the hydroxyproline weight normalized to the DNA weight (w/w) also showed a stable value, with an average of ∼46 from day 0 to day 2. However, their collagen content increased to 65 on day 10 and decreased back to 51 on day 17. A decrease in collagen content on day 17 was also observed in this study. Aside from the high collagen content normalized to the DNA weight (w/w) found on day 0 (caused by the low cell density on day 0), the collagen content was quite stable in this study, remaining at a value of 63 prior to its abrupt decrease to 46 on day 17.
Based on the results of this study, we conclude that healthy cartilage responds well to the blunt trauma (exerted during fixation by the clamp) and the abrasive, thermal, and lacerative trauma (exerted during cutting with the saw and hollow punch) sustained by the adjacent cartilage during explantation. After the two-day equilibration period, the cartilage remained stable for up to 10 days of culture. Serum-supplemented culture medium, despite its potential for adverse effects (cell outgrowth, which did not occur in this study),27 did help the cartilage respond positively to the sustained trauma. The degradation of collagen on day 17, albeit not to diseased levels, showed that explanted cartilage could potentially develop into degenerated cartilage. This has applications in pharmacological and pathophysiological fields.15
CONCLUSIONSHealthy cartilage metabolism was assessed by studying changes in GAG, collagen, and DNA content over 17 days of culture. In general, cartilage metabolism was found to be relatively stable for a culture period of up to 10 days if the explant was given two days to equilibrate. However, the collagen fibers in the cartilage begin to degrade if the culture period is longer than ten days, which means that the cartilage could potentially develop into degenerated cartilage. It is apparent that tissue stability and remodeling dynamics are complex and influenced by many parameters. In this study, we sought to report the dynamics of cartilage metabolism in the context of a generalized culture process; future studies should aim to elucidate the factors that affect the long-term culture characteristics of bovine articular cartilage.
We would like to thank Vincent Murphy for his invaluable comments and Adhli Iskandar Putera bin Hamzah for his efforts in procuring the bovine feet. This research was funded by the Institute of Research Management and Monitoring, University of Malaya, Malaysia.