Thoracic aortic aneurysms (TAAs) represent one-third of the hospitalizations for aortic diseases. The prevalence rate depends on the definition of the normal size of the aorta, which is quite variable, depending on the population studied. The aim of this study was to evaluate the characteristics of the thoracic aorta of Brazilian smokers, identifying the normal size of the aorta, presence of anatomical variations, and prevalence of TAA.
MATERIALS AND METHODS:A total of 711 patients underwent radiological evaluation with low-dose computed tomography (CT) from January 2013 to July 2014 with the initial objective of lung nodule tracking. Two examiners evaluated these images, and measurements of maximum and serial diameters were performed manually in true orthogonal planes. Serial diameter measurements were taken every 2 cm in the ascending aorta and 5 cm in the descending segment. We searched for anatomical variations, aortic arch type, and correlations between anatomical characteristics, sex, body mass index, and body surface area (BSA).
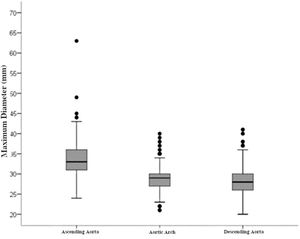
RESULTS:The maximum diameters were 33.61 (standard deviation [SD] 3.88), 28.66 (SD 2.89), and 28.36 mm (SD 3.09) for the ascending segment, aortic arch, and descending segment, respectively. A positive correlation was found between male sex, age, and BSA and aorta diameter. The bovine arch was the most common variation of the aortic arch type, and we found one (0.14%) case of TAA.
CONCLUSIONS:This study with low-dose CT allowed the determination of the mean diameters of the ascending aorta, aortic arch, and descending aorta in Brazilian smokers and TAA prevalence.
Aortic aneurysms are the third leading cause of vascular disease in the United States. Thoracic aortic aneurysms (TAAs) represent one-third of the hospitalizations for aortic diseases (1). TAAs are classified according to their anatomical location: ascending aorta aneurysms (most common), aortic arch aneurysms, descending TAAs, and thoracoabdominal aneurysms (2).
TAAs and infrarenal abdominal aortic aneurysms share the same risk factors: smoking, systemic hypertension, and dyslipidemia (3). TAAs are often asymptomatic, which hinders their diagnosis, present slow growth, are strongly related to congenital and degenerative diseases (4), and can cause severe complications such as upper limb ischemia, carotid and vertebral embolization, and, the most feared of all, rupture.
The surgical treatment of aneurysms aims to reduce the risk of these complications and to treat symptoms, when they exist, to avoid rupture. However, surgical treatment is not risk-free (5,6). Thus, it is only recommended when the maximum diameter of the aneurysm, in the descending thoracic aorta, is greater than 6 cm (7). Above these diameters, the risks of surgery are lower than the risk of rupture, justifying treatment.
Several studies have sought to identify the actual incidence of TAAs; however, the incidence rate depends on the definition of the normal size of the aorta, which is quite variable, depending on the population studied. Hence, it is important to first determine the characteristics of a normal population to evaluate the incidence of TAAs. Recently, two studies were conducted to analyze the average size of all segments of the thoracic aorta using chest computed tomography (CT) in patients without risk factors for TAAs and thus evaluate the prevalence of TAAs in these populations (8,9). Itani et al., in Japan, in 2002 (8), used the population's standard deviation (SD) to define an abnormality value; values above three SDs were considered abnormal. Thus, they found a prevalence of 0.16%. Kälsch et al., in 2013, in Germany, defined any dilatation of the thoracic aorta with a diameter greater than 5 cm as a TAA, resulting in a prevalence of 0.34% (9).
In Brazil, this type of study has not yet been performed and is relevant both internally to demonstrate the local anatomical conditions and to the rest of the world to demonstrate the difference in relation to other countries and the possible need for individualized population studies.
The aim of this study was to evaluate the characteristics of the thoracic aorta of Brazilian smokers, identifying the normal size of the aorta, presence of anatomical variations in the supra-aortic trunks, and prevalence of TAAs.
MATERIALS AND METHODSA total of 711 volunteers aged between 55 and 74 years, smokers (tobacco use 30 packs/year or more), and without signs or symptoms of aortic diseases were studied. All patients underwent radiological evaluation with low-dose CT from January 2013 to July 2014 with the initial objective of lung nodule tracking. Some of them were followed up, but at this moment, we analyzed only the first images. The anthropometric characteristics are presented in Table 1. The included patients were in the sixth decade of life and had mild overweight and a similar distribution between sexes, with an average body surface area (BSA) of 1.83 m2.
Patient characteristics.
| Age (years) | |
| Mean (SD) | 60.9 (4.7) |
| Median (Q1; Q3) | 60 (57; 64) |
| Range | 54-74 |
| Sex | |
| Male | 352 (49.5%) |
| Female | 359 (50.5%) |
| Weight (kg) | |
| Mean (SD) | 73.0 (15.2) |
| Median (Q1; Q3) | 71 (62; 81) |
| Range | 35-131 |
| Height (cm) | |
| Mean (SD) | 166.4 (9.1) |
| Median (Q1; Q3) | 167 (160; 173) |
| Range | 141-193 |
| Body mass index (kg/m2) | |
| Mean (SD) | 26.3 (4.7) |
| Median (Q1; Q3) | 25.8 (23.3; 28.7) |
| Range | 15.6-49.8 |
| Body surface area (m2) | |
| Mean (SD) | 1.83 (0.22) |
| Median (Q1; Q3) | 1.81 (1.67; 1.97) |
| Range | 1.21-2.55 |
SD: standard deviation.
The tests were performed using a single device (Toshiba Aquilion 64 CFX CT, Toshiba Medical Systems Corporation, Otawara, Tochigi, Japan) and a low radiation dose technique (120 kV and a maximum of 15 mA with a 1×1 mm collimation). The images were stored in a Picture Archiving and Communication System (PACS) in the Digital Imaging and Communications in Medicine (DICOM) format.
These images were evaluated by two examiners, and measurements were performed manually using Horos version 3.2.0 (Horos Project, Geneva, Switzerland). At each segment, diameter measurements were taken of the thoracic aorta (ascending aorta, aortic arch, and descending aorta) in true orthogonal planes, corrected by the central axis, by a single examiner.
Then, serial measurements were made at precisely defined points. In the ascending aorta, diameter measurements were taken every 2 cm with the origin immediately proximal to the brachiocephalic trunk. In the descending aorta, serial measurements were performed every 5 cm with the origin immediately distal to the origin of the left subclavian artery.
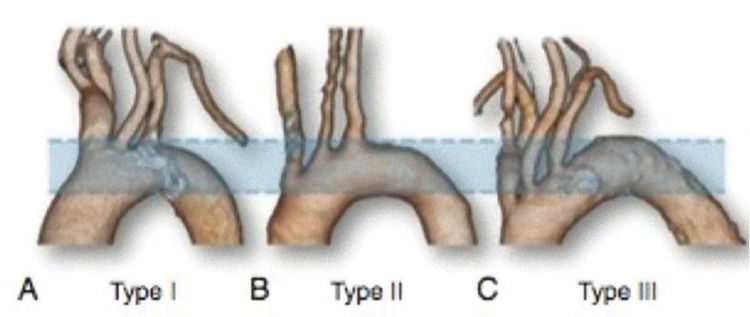
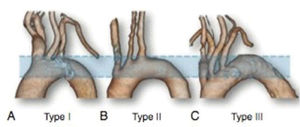
The most frequent anatomical variations were evaluated: the presence of a common origin between the right carotid artery and the brachiocephalic trunk (bovine arch) and the presence of an abnormal origin of the left vertebral artery and aberrant left subclavian artery. Aortic arch types were classified as follows: type 1, when the origin of the supra-aortic trunk is found in an imaginary plane above the greater curvature of the aorta; type 2, when one of the vessels originates in a plane between the greater and lesser curvature of the aorta; and type 3, when one of the vessels originates proximal to the plane drawn in the lesser curvature of the aorta (Figure 1). In addition, anatomical characteristics were correlated with anthropometric characteristics, body mass index (BMI), and BSA.
To identify the importance of each variable in determining the diameter value for each segment, we performed multiple linear regression with the following parameters: sex, age, and average BSA. Based on this analysis, we obtained the expected measures for each segment, stratified by sex and age group.
This study was approved by the institution's ethics committee and exempt from informed consent.
RESULTSFor each patient, we performed, at least, six measures of aortic diameters: two in the ascending segment, one in the aortic arch, and three in the descending aorta. This resulted in 4,266 diameter measurement points and a total of 8,532 measurements.
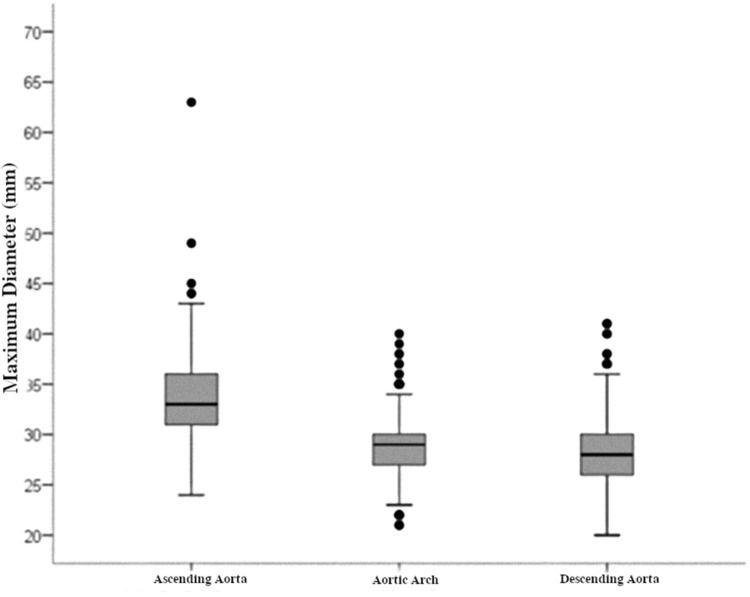
The maximum diameters of each segment are shown in Figure 2. The largest arterial diameter was observed in the ascending segment, at 33.61 mm (SD, 3.88). In the aortic arch and the descending aorta, we found smaller diameters (28.66 mm [SD 2.89] and 28.36 [SD 3.09], respectively).
Analysis of the correlation between values and anthropometric characteristics showed a positive correlation between male sex, age, and BSA with aorta diameter, with a stronger correlation for BSA and the descending aorta (Table 2).
Correlations between aortic diameter, age, and body surface area.
| Variables | Total | Female | Male |
|---|---|---|---|
| Age | n=711 | n=359 | n=352 |
| Ascending aorta | 0.240 (p<0.001) | 0.259 (p<0.001) | 0.172 (p=0.001) |
| Aortic arch | 0.252 (p<0.001) | 0.157 (p=0,003) | 0.281 (p<0.001) |
| Descending aorta | 0.322 (p<0.001) | 0.190 (p<0,001) | 0.386 (p<0.001) |
| Body surface area | n=707 | n=358 | n=349 |
| Ascending aorta | 0.350 (p<0.001) | 0.207 (p<0.001) | 0.263 (p<0.001) |
| Aortic arch | 0.376 (p<0.001) | 0.288 (p<0.001) | 0.237 (p<0.001) |
| Descending aorta | 0.403 (p<0.001) | 0.286 (p<0.001) | 0.203 (p<0.001) |
Pearson correlation (p value).
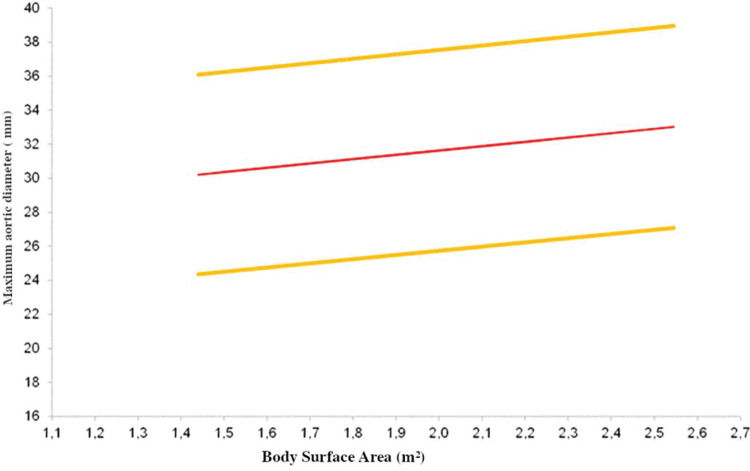
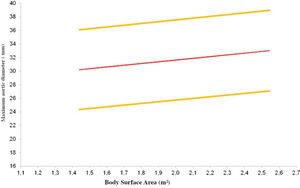
Multiple linear regressions confirmed that age, BSA, and male sex were good predictors of aortic size in the descending segment, as shown in Table 3. The equations used to calculate the diameter of each segment are shown in Table 4, and the expected aortic diameters for each segment are represented in Figure 3.
Predictors of descending aorta diameter.
| Predictor | Unstandardized coefficient (standardized) | Standard error | p value |
|---|---|---|---|
| Age | 0.108 (0.166) | 0.029 | <0.001 |
| Male sex | −7.280 (−1.180) | 2.479 | 0.003 |
| Body surface index | 3.833 (0.272) | 0.491 | <0.001 |
| Age and male sex | 0.149 (1.494) | 0.040 | <0.001 |
| Constant | 13.811 | 1.982 | <0.001 |
Linear regression equations for each segment.
| Aortic segment | Linear regression equation |
|---|---|
| Ascending | Maximum diameter=11.849+0.202 × age+3.583 (for male sex)+4.829 × BSA − 0.038 × age (for male sex) |
| Arch | Maximum diameter=15.060+0.095 × age − 4.417 (for male sex)+3.988 × BSA+0.088 × age (for male sex) |
| Descending | Maximum diameter=13.811+0.108 × age − 7.280 (for male sex)+3.833 × BSA+0.149 × age (for male sex) |
Anatomical variations were observed in 81 (11.4%) patients, and the bovine arch was the most common variation. Among the aortic arch types, type 1 was the most common (Table 5).
Finally, we found only one (0.14%) aneurysm in the descending aorta.
DISCUSSIONTAA is a silent disease of increasing incidence and high morbidity and mortality, the prognosis of which depends substantially on the size of the aorta in each segment. In the past, analyses of normal vessel dimensions were performed based on angiography, ultrasound (10), and magnetic resonance imaging (11). With the increased availability of tomography, studies to identify the anatomical features of the aorta have been performed. In recent years, several studies have been conducted with this aim in North America (2,12,13), (14) Europe (9), and the East (8,15). The objective of our study was to analyze the dimensions of the aorta in Brazil and evaluate the impact of BMI, BSA, and age.
Currently, there is increasing concern about the deleterious effects of radiation. Thus, the use of tomography exams with low radiation doses, as performed in our study, is gaining importance. Wolak et al. (12) were the first to evaluate, on a large scale, the dimensions of large vessels with the use of tomography without contrast. Their data were comparable to those of a contrasted series and showed a high interobserver correlation. Thus, we can infer that the modern protocols do not require contrast and high doses of radiation to measure aortic diameters.
In our study, we used a sample previously selected for the screening of lung diseases (16). Thus, our study included patients in an older age group, similar to two studies conducted in Japan and Korea, which also used samples previously selected for lung neoplasia screening (8,15).
The demographic characteristics of our sample were similar to those in a national study that evaluated causes of death in Brazilian patients with aortic aneurysms (17), with no predominance of any sex and with a slightly higher than average age than American (2) and German (9) populations.
The maximum diameter of the ascending aorta was similar to that reported for two American series that used patients undergoing coronary disease control exams (12,18) and was lower than that reported for a large population study (2). This difference could be due to selection bias. A review which compared the works of Rogers, Wolak and Mao enrolled younger patients who consequently presented with fewer comorbidities and smaller vessels.
When compared with the mean diameter of the ascending aorta in non-American populations, the mean diameter of the ascending aorta in Brazilians was 1 mm greater than that found in Japanese patients (8) (younger patients) and 5 mm smaller than that found in a German population (9).
The aortic arch diameter tends to be less evaluated in series because its evaluation demands a more arduous methodology. Even so, we could compare our results with those reported in a study by Hager et al. (19) in German patients, which showed a slightly higher average diameter than that in Brazilians. However, the diameter of the descending aorta was higher than that reported by the majority of series (8,9,12,15), except for the Japanese series (8), which can be explained by the mean age of our patients, which was 60.9 years, compared to other studies with populations younger than 60 years (9,19).
Therefore, the diameter of the ascending aorta was smaller in Europeans and larger in Asians, while the diameter of the descending aorta had opposite characteristics.
There was a significant difference in measurements between sexes, with larger diameters in men in all segments, based on data previously demonstrated by other studies (9,12) and reaffirmed in our study.
The relationship between anthropometric data and vessel dimensions is already known. BMI, age, and BSA were related to the diameter of the thoracic aorta, with correlations similar to European and Asian studies. Thus, we sought to correlate aortic diameters with BMI and mean BSA, which is more accurate than BMI in evaluating the correlation between body measurements and aortic diameters (20).
Our patients had a mean BMI of 26.28, which is compatible with Brazilian data, which show a high incidence of overweight status, especially in age groups older than 60 years. The population in our study showed a lower BSA than most studies (9,12,19), with the exception of a Korean study (15). These variations confirm the need for careful evaluation when comparing data from different populations.
Keeping in mind that our population has a smaller BSA than others and that there was a correlation between BSA and aortic diameter in three segments (ascending aorta, trunk, and descending aorta), one would expect proportionally smaller diameters compared to those reported in other studies. We think that the high rate of smoking and the consequences of its use on the arterial wall led to an increase in aortic diameter.
The diameter is also influenced by age, which showed a significant correlation in all segments, especially in the descending aorta, as observed by Rogers et al. and Itani et al. (2,8). We know that there is a greater concentration of elastic tissue in the thoracic aorta as it approaches the diaphragm. Over time, degeneration of these fibers occurs, which leads to a progressive increase in the vessel by losing its elastic characteristics, becoming less compliant.
Because there is considerable phenotypic variation among the populations (Europeans, for example, have greater stature than Eastern populations), we performed multiple linear regression that resulted in a formula to find the expected diameter of each aortic segment as a function of BSA, BMI, and age range.
We observed an incidence of anatomical variations in the supra-aortic trunks in 11.4% of the sample. As in other studies (21,22), we found that the most common variation was the bovine arch, present in 9.6% of our sample. Mylonas (23) found an increased risk of aortic diseases in the presence of a bovine trunk; however, we found no relationship between anatomical variation and the presence of aneurysms.
Our study has some limitations. We are the first group to accurately assess the anatomical features of the aorta of Brazilians. These analyses were performed in non-contrast low-dose exams, which resulted in lower morbidity. In contrast to other series, which made two measurements in the axial plane (8,9,12,18), we used precisely defined sites in several aortic segments and performed all corrective measurements for the true axis of the vessel, which is the most accurate method (24) and recommended by the guidelines.
This resulted in 4,200 evaluated diameters that led us to find, with great accuracy, the real diameter of each segment (ascending aorta, aortic arch, and descending aorta).
In addition, the sample had a high prevalence of smokers and elderly individuals, which are factors usually related to larger aortic dimensions. However, they are the most frequent patients with TAAs and are therefore a good indicator of the measurements of patients at risk for developing the disease. In addition, the evaluation of the proximal segment of the aorta close to the sinus, an area of high tissue density that was impaired by the absence of contrast, prevented accurate measurements of the extent of the ascending aorta segment.
We believe that with technological evolution, using artificial intelligence and robotic analysis mechanisms, we will be able to evaluate large volumes of CT data and have more significant anatomical data from global populations in the near future.
CONCLUSIONSIn elderly Brazilian smokers, who are part of a population at greatest risk for aneurysms, the mean diameters of the ascending aorta, aortic arch, and descending aorta were 33.6 mm, 28.7 mm, and 28.4 mm, respectively.
Anatomical variations were observed in 81 (11.4%) patients, with bovine arch being the most common, and TAA prevalence was 0.14%.
AUTHOR CONTRIBUTIONSLembrança L, Teivelis MP, Tachibana A, dos Santos RS were responsible for the research, manuscript development, data collection and manuscript writing. Joo RW and Zippo E were responsible for the data collection. Wolosker N was responsible for the manuscript development and approval.
No potential conflict of interest was reported.