The latest published studies show an increased incidence of thyroid cancer worldwide. The aim of this study was to analyze the changes in the incidence of thyroid cancer in Navarra and its clinical presentation regarding sex, histological subtype and size over the last 25 years.
MethodsThyroid cancer incidence rates were calculated on the basis of data from the Cancer Registry of Navarra during 1986–2010. Clinical data were obtained from the historical cohort of the Hospital Registry of Cancer of Navarra, which includes all the new cases of differentiated thyroid carcinoma diagnosed and treated in the public health network of this Community in that period.
ResultsThe overall incidence of thyroid cancer in Navarra increased over the last 25 years, with an increase in the adjusted rate in men from 2.24 (1986–1990) to 5.85 (2006–2010) per 100,000 population/year (p<0.001) and in women from 9.05 to 14.04, respectively (p<0.001). This increase occurs only in papillary carcinoma. The clinical characteristics of 739 patients with differentiated thyroid cancer were studied. The mean age at diagnosis increased over the years and the predominance of women (about 80%) remains stable. Mean tumor size decreased over the five-year periods from 30.9 to 22.5mm (p<0.001), the proportion of microcarcinomas (T1a) increased from 8.8% to 30% (p<0.001) and, despite this increase, there were no statistical differences in the TNM stage at diagnosis during the study period. The distribution of histological variants of papillary and follicular carcinoma did not change over 25 years.
ConclusionsDuring the period studied, the incidence of thyroid cancer increased in Navarra in both sexes. The increase occurred only in papillary carcinoma, without changes in the distribution of his histological variants. The increase in the proportion of T1a tumors is remarkable, but the TNM stage distribution was maintained. These results suggest an increase in the diagnosis of thyroid microcarcinomas due to changes in clinical practice, without ruling out a real increase in the incidence of papillary carcinoma in Navarra.
En los últimos estudios publicados se observa un aumento de la incidencia de cáncer de tiroides a nivel mundial. El objetivo del presente estudio fue analizar los cambios en la incidencia de cáncer de tiroides en Navarra, y su presentación en cuanto a sexo, subtipo histológico y tamaño a lo largo de los últimos 25 años.
MétodosSe calcularon las tasas de incidencia de cáncer de tiroides a partir de los datos del Registro de Cáncer de Navarra durante el periodo 1986–2010. Los datos clínicos se obtuvieron de la cohorte histórica del Registro Hospitalario de Cáncer de Navarra, que incluye todos los nuevos casos de carcinoma diferenciado de tiroides diagnosticados y tratados en la red sanitaria pública de esta comunidad en dicho periodo.
ResultadosLa incidencia global de cáncer de tiroides en Navarra ha aumentado en estos 25 años con un incremento en la tasa ajustada en varones de 2,24 (1986–1990) a 5,85 (2006–2010) por 100.000 habitantes/año (p<0,001), y en mujeres de 9,05 a 14,04, respectivamente (p<0,001). Este aumento se produjo únicamente a expensas del carcinoma papilar. En el ámbito hospitalario, se estudiaron 739 pacientes con cáncer diferenciado de tiroides. La edad media al diagnóstico aumentó a lo largo de los años y el predominio de mujeres (alrededor del 80%) se mantuvo estable. El tamaño tumoral medio disminuyó a lo largo de los quinquenios de 30,9 a 22,5mm (p<0,001), la proporción de microcarcinomas (T1a) aumentó de 8,8% a 30% (p<0,001) y, a pesar de este aumento, no hubo diferencias estadísticas en el estadio TNM al diagnóstico durante el periodo de estudio. La distribución de las variantes histológicas de carcinoma papilar y folicular no se modificó a lo largo de los 25 años.
ConclusionesDurante el período estudiado la incidencia de cáncer de tiroides ha aumentado en Navarra en ambos sexos. El aumento se ha producido a expensas del carcinoma papilar, sin cambios en la distribución de las variantes histológicas. Destaca el aumento en la proporción de tumores T1a, pero se mantiene la distribución por estadio TNM. Estos resultados sugieren un incremento del diagnóstico de microcarcinomas tiroideos por cambios en la práctica clínica, sin descartar además un incremento real de la incidencia del carcinoma papilar en Navarra.
Thyroid cancer is the most frequent endocrine malignancy, most such neoplasms (over 90%) being differentiated tumors derived from thyroid follicular cells. In recent decades there has been a gradual increase in the incidence of thyroid cancer in many countries and regions throughout the world. The incidence rates have increased rapidly in southern Europe, the United States, Australia and particularly in Korea, but only moderately so in northern Europe and Japan.1–6 This increase is almost exclusively due to papillary tumors, with a particularly notorious increase in microcarcinomas (tumors measuring 1cm or less in size), which represent over 43% of the total in some series.7 An increase in the incidence of thyroid carcinoma has also been detected in some regions in Spain.8,9
Some studies have suggested that there has been no genuine increase in incidence, and that the mentioned observations are due to changes in clinical practice, characterized by an increasingly widespread use of imaging techniques that make it possible to incidentally detect small tumors in their early stages, and to the increase in total thyroidectomies versus other surgical techniques.10,11 Hence some authors have warned against a situation of overdiagnosis and overtreatment in such very low risk tumors.12–14
Other studies have observed a very real increase in the incidence and prevalence of thyroid cancer, mainly at the expense of papillary tumors. Different explanations for these observations have been proposed, such as the generalized iodization of the population, exposure to ionizing radiation, cervical radiotherapy in childhood, thyroid autoimmune conditions, certain nutritional factors, environmental toxins, or genetic syndromes.15,16
The present study was carried out to evaluate the changes in the incidence of thyroid cancer and in the clinical characteristics of differentiated carcinoma in regard to size, histological subtype and initial stage, during the period 1986–2010 in Navarre (Spain).
Material and methodsThe incidence of thyroid cancer was calculated from the Navarre Cancer Registry, which covers the entire resident population of the region (approximately 640,000 inhabitants in 2010). The rates per 100,000 inhabitants per year for males and females were estimated, adjusting for age and taking as reference the European standard population, with a 95% confidence interval (95%CI) per gender and period.
Of the total thyroid tumors, approximately 90% corresponded to differentiated thyroid carcinoma (DTC).17 The clinical study was carried out using the database of the Navarre Hospital Cancer Registry, comprising 739 cases of DTC diagnosed, operated upon and followed-up on in the public health centers of Navarre during the period 1986–2010. The data were accessed in observance of current legislation regarding patient confidentiality (Orden Foral 96/2006, of 11 October), with the approval of the Clinical Research Ethics Committee. The registry documented patient age, gender, date of diagnosis, form of diagnosis, tumor size, histological subtype, the presence of adenopathies, distant metastases and initial stage.
The histological classification was based on the World Health Organisation (WHO) classification,18 using two principal categories: papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC). The latter in turn includes the minimally and widely invasive variants, the clear cell variant, and Hürthle cell carcinoma (or oncocytic variant). The histological variants of papillary carcinoma were grouped into three categories for posterior analysis: (a) classical papillary carcinomas (this group moreover includes those other variants with a low incidence and similar clinical aggressiveness, such as cribiform-morular, oxyphilic or clear cell carcinomas); (b) follicular variant papillary carcinomas; and (c) the more aggressive histological variants, including the diffuse sclerotizing, solid-trabecular, tall cell and column cell subtypes.
All tumors were studied at the time of diagnosis, in accordance with the 6th Edition TNM classification of the American Joint Committee on Cancer 2002.19 Those tumors measuring 1cm or less in diameter, located within the thyroid gland and without lymph node involvement or metastasis were classified as microcarcinomas or stage T1a tumors.
The data were analyzed using the SPSS version 20.0 and STATA 10.0 statistical packages. We calculated the annual percentage changes in the course of the period 1986–2010 adjusted for age, together with the corresponding p-value for the thyroid cancer incidence rates based on Poisson regression analysis. The qualitative variables were reported as frequency distributions for each of the categories, and the quantitative variables as the mean and standard deviation. The study period was divided into five 5-year intervals. The change in distribution of the different types of thyroid cancer over time was assessed using the chi-squared test. The comparison of means of independent samples was carried out using analysis of variance (ANOVA). A statistical significance level of 5% was accepted (p<0.05).
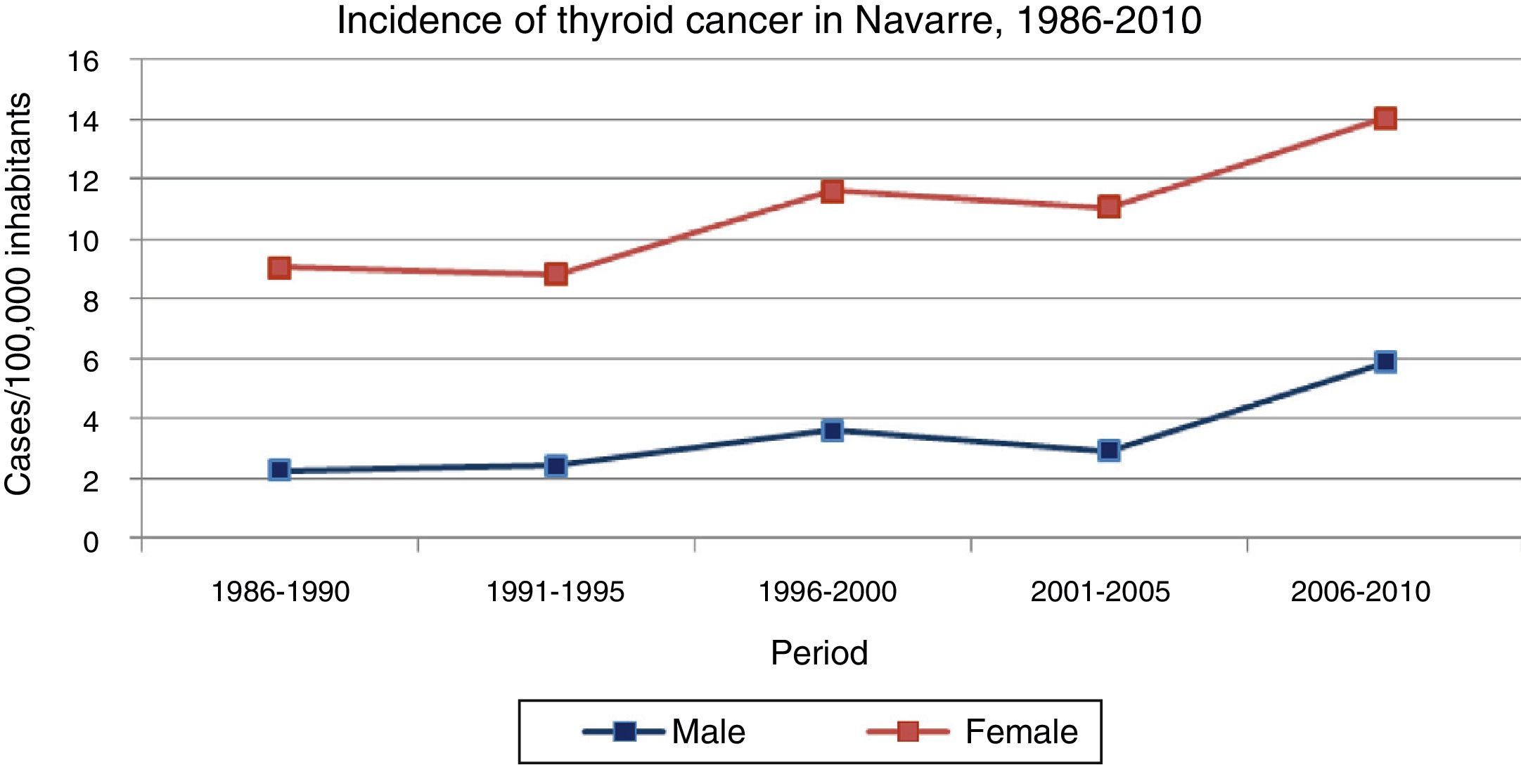
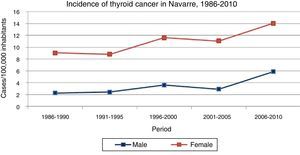
ResultsThe incidence of thyroid cancer in Navarre was seen to increase in the course of the study period in both genders (Fig. 1) (Table 1), with a higher incidence among females at all times. Specifically, the incidence among females increased from 9.05 to 14.04 cases per 100,000 inhabitants/year from the first to the last 5-year interval (p<0.001), while in males the incidence increased from 2.24 to 5.85 cases per 100,000 inhabitants/year in that same period (p<0.001). The annual percentage change was 4.7 (2.9–6.6) in males and 2.5 (1.6–3.5) in females.
Incidence of thyroid cancer in Navarre, 1986–2010.
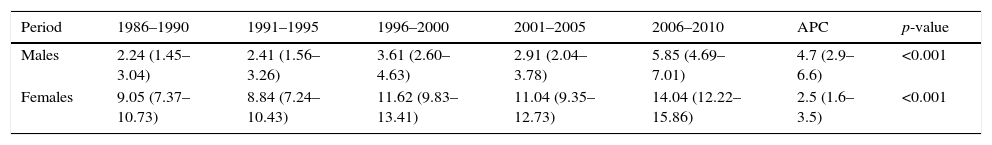
| Period | 1986–1990 | 1991–1995 | 1996–2000 | 2001–2005 | 2006–2010 | APC | p-value |
|---|---|---|---|---|---|---|---|
| Males | 2.24 (1.45–3.04) | 2.41 (1.56–3.26) | 3.61 (2.60–4.63) | 2.91 (2.04–3.78) | 5.85 (4.69–7.01) | 4.7 (2.9–6.6) | <0.001 |
| Females | 9.05 (7.37–10.73) | 8.84 (7.24–10.43) | 11.62 (9.83–13.41) | 11.04 (9.35–12.73) | 14.04 (12.22–15.86) | 2.5 (1.6–3.5) | <0.001 |
Rates adjusted for age and taking as reference the European standard population (cases per 100,000 inhabitants/year) (95%CI).
APC: annual percentage change
Source: Navarre Cancer Registry.
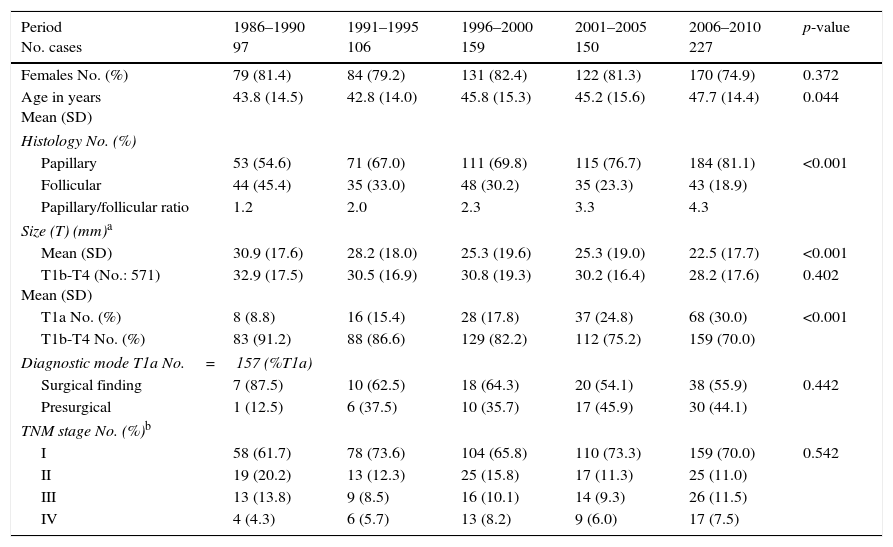
The clinical characteristics of the 739 patients with DTC diagnosed in Navarre in the period 1986–2010 are shown in Table 2. Females accounted for 79.7% and males 20.3%, and this gender distribution remained stable over time. The mean patient age (standard deviation [SD]) at the time of diagnosis was 46.6 (14.7) years, and showed a slight but statistically significant increase over time (from 43.8 to 47.7 years).
Characteristics of the patients with differentiated thyroid carcinoma in Navarre, 1986–2010.
| Period No. cases | 1986–1990 97 | 1991–1995 106 | 1996–2000 159 | 2001–2005 150 | 2006–2010 227 | p-value |
|---|---|---|---|---|---|---|
| Females No. (%) | 79 (81.4) | 84 (79.2) | 131 (82.4) | 122 (81.3) | 170 (74.9) | 0.372 |
| Age in years Mean (SD) | 43.8 (14.5) | 42.8 (14.0) | 45.8 (15.3) | 45.2 (15.6) | 47.7 (14.4) | 0.044 |
| Histology No. (%) | ||||||
| Papillary | 53 (54.6) | 71 (67.0) | 111 (69.8) | 115 (76.7) | 184 (81.1) | <0.001 |
| Follicular | 44 (45.4) | 35 (33.0) | 48 (30.2) | 35 (23.3) | 43 (18.9) | |
| Papillary/follicular ratio | 1.2 | 2.0 | 2.3 | 3.3 | 4.3 | |
| Size (T) (mm)a | ||||||
| Mean (SD) | 30.9 (17.6) | 28.2 (18.0) | 25.3 (19.6) | 25.3 (19.0) | 22.5 (17.7) | <0.001 |
| T1b-T4 (No.: 571) Mean (SD) | 32.9 (17.5) | 30.5 (16.9) | 30.8 (19.3) | 30.2 (16.4) | 28.2 (17.6) | 0.402 |
| T1a No. (%) | 8 (8.8) | 16 (15.4) | 28 (17.8) | 37 (24.8) | 68 (30.0) | <0.001 |
| T1b-T4 No. (%) | 83 (91.2) | 88 (86.6) | 129 (82.2) | 112 (75.2) | 159 (70.0) | |
| Diagnostic mode T1a No.=157 (%T1a) | ||||||
| Surgical finding | 7 (87.5) | 10 (62.5) | 18 (64.3) | 20 (54.1) | 38 (55.9) | 0.442 |
| Presurgical | 1 (12.5) | 6 (37.5) | 10 (35.7) | 17 (45.9) | 30 (44.1) | |
| TNM stage No. (%)b | ||||||
| I | 58 (61.7) | 78 (73.6) | 104 (65.8) | 110 (73.3) | 159 (70.0) | 0.542 |
| II | 19 (20.2) | 13 (12.3) | 25 (15.8) | 17 (11.3) | 25 (11.0) | |
| III | 13 (13.8) | 9 (8.5) | 16 (10.1) | 14 (9.3) | 26 (11.5) | |
| IV | 4 (4.3) | 6 (5.7) | 13 (8.2) | 9 (6.0) | 17 (7.5) | |
Source: Hospital Cancer Registry.
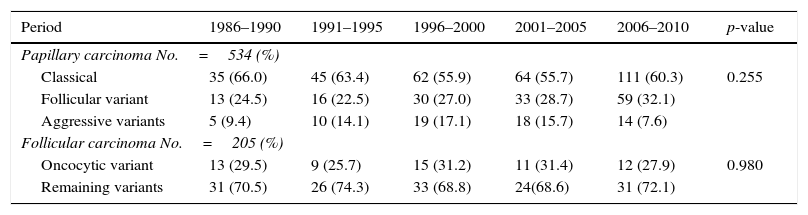
Most of the DTCs (72.3%) corresponded to papillary carcinoma, which represented the most frequent histological type, while follicular carcinoma accounted for 27.7%. An increase was noted in the frequency of PTC in the course of the 5-year intervals (p<0.001), with a gradual increase in the PTC/FTC ratio to 4.3 in the last interval.
With regard to the histological variants of PTC, the most frequent subtype was classical papillary carcinoma (59.4%), followed by the follicular variant (28.3%) and the aggressive subtypes (12.4%). The distribution of these variants remained stable over time and without significant differences (p=0.255). In the case of FTC, the proportion between Hürthle cell carcinoma and the remaining histological variants was found to be similar in the course of the 5-year intervals (p=0.980) (Table 3). During these 25 years there was a gradual increase in the number of microcarcinomas (T1a tumors) from 8.8% of the total DTCs in the first 5-year interval (1986–1990) to 30% during 2006–2010 (p<0.001) (Table 2).
Distribution of the histological subtypes of papillary and follicular carcinoma in Navarre, 1986–2010.
| Period | 1986–1990 | 1991–1995 | 1996–2000 | 2001–2005 | 2006–2010 | p-value |
|---|---|---|---|---|---|---|
| Papillary carcinoma No.=534 (%) | ||||||
| Classical | 35 (66.0) | 45 (63.4) | 62 (55.9) | 64 (55.7) | 111 (60.3) | 0.255 |
| Follicular variant | 13 (24.5) | 16 (22.5) | 30 (27.0) | 33 (28.7) | 59 (32.1) | |
| Aggressive variants | 5 (9.4) | 10 (14.1) | 19 (17.1) | 18 (15.7) | 14 (7.6) | |
| Follicular carcinoma No.=205 (%) | ||||||
| Oncocytic variant | 13 (29.5) | 9 (25.7) | 15 (31.2) | 11 (31.4) | 12 (27.9) | 0.980 |
| Remaining variants | 31 (70.5) | 26 (74.3) | 33 (68.8) | 24(68.6) | 31 (72.1) | |
Source: Hospital Cancer Registry.
The mean tumor size was 24mm (SD=18), and it gradually decreased over time from 30.9mm (SD=17.6) to 22.5mm (SD=17.7) in the period from the first to the last 5-year interval studied (p<0.001). However, on excluding the microcarcinomas, no significant change in tumor size at the time of diagnosis was observed (p=0.402) (Table 2).
In the 157 microcarcinomas of our series, the proportion of cases that were diagnosed using imaging techniques before surgery increased from 12.5% to 44.1% between the first and last 5-year interval, with a consequent decrease in the proportion of occult tumors detected at the time of thyroidectomy performed for some other benign disease condition. However, this change was not statistically significant (Table 2).
With regard to TNM staging at the time of diagnosis, 69.3% of the tumors were in stage I, 13.5% in stage II, 10.6% in stage III, and 6.7% in stage IV. There were no statistically significant differences in tumor distribution by stages in the course of the 5-year intervals studied (p=0.542) (Table 2).
DiscussionThe present study evaluated the evolution of the incidence of thyroid cancer and the clinical characteristics of DTC in Navarre (Spain) over a 25-year period. The data were obtained from the Navarre Cancer Registry for the estimation of the population-based rates, and from the Hospital Cancer Registry for the analysis of the clinical variables of the cases diagnosed and treated in the setting of the Navarre public health network.
We observed an increase in tumor incidence in both genders, rates of 14.04 and 5.85 cases per 100,000 inhabitants/year in females and males, respectively, being reached in the last 5-year interval studied (2006–2010). These rates are the highest of those published in different regions in Spain,8,9 and are greater than the estimated thyroid cancer incidence for 2012 in Europe.20 The observed increase in thyroid cancer in our setting is due only to the increase in papillary carcinoma, with no increase in follicular carcinoma. These findings are consistent with those of studies in other populations that likewise confirm an increase in papillary thyroid carcinoma both in European countries4–6 and elsewhere throughout the world.1,7,8
A factor that may have contributed to the observed increase in papillary carcinoma in Navarre is the change in the iodization status of the population. In effect, iodine deficiency zones were detected in Navarre in the 1980s and 1990s,21 though this region has since become iodine-sufficient.22 Nevertheless, the association between iodine intake and thyroid cancer is subject to controversy.23
In our series we have not found the increase in PTC to imply a change in the distribution of the different histological variants, which has remained constant over time. Other authors have described a progressive increase in the follicular variant of papillary carcinoma, with regard to both the encapsulated and the infiltrating form,24 though not all studies have recorded such an increase.25 The recent modification of the histopathological classification of the encapsulated follicular variant of papillary carcinoma may result in a change in the proportions of the different variants of PTC, and will facilitate advances in the study of these tumors and their evolution.26
With regard to tumor size at the time of diagnosis, the mean size was seen to decrease gradually at the expense of an increase in the number of microcarcinomas, since on excluding the stage T1a lesions from the analysis, the average tumor size was not seen to have varied over the 25-year study period. In our series, microcarcinomas represented 30% of all the tumors in the last 5-year interval. The results obtained are consistent with those of other studies, and in our series can be explained by a larger number of thyroidectomies resulting in the identification of a greater number of occult carcinomas in the surgical pieces; an increase in the incidental diagnosis of tumors secondary to the use of imaging techniques for other indications (supraaortic trunk echo-Doppler, magnetic resonance imaging, computed tomography, positron emission tomography); and the widespread use of cervical ultrasound and puncture-aspiration procedures since the 1990s. Based on tumor size at the time of diagnosis, it has been estimated that more than 50% of the increase in the incidence of papillary cancer since 1980 is due to overdiagnosis.27 Since such tumors may be regarded as clinically irrelevant, the hazards of the overtreatment of such patients must be taken into account. The new clinical guidelines therefore recommend limiting study to those intrathyroid nodules measuring over 1cm in size. This may cause the proportion of microcarcinomas to decrease in the future in comparison with the current situation.28
The main strengths of our study are the long time period analyzed, the inclusion of relevant clinical variables, and the analysis of the thyroid cancer incidence rates using population based data.
In conclusion, the incidence of thyroid cancer increased in Navarre during the period 1986–2010. This increase is exclusively attributable to papillary carcinoma, with no changes in the distribution of the histological variants. The proportion of microcarcinomas (stage T1a lesions) has increased, though without changes in the TNM-based classification at the time of diagnosis. The increase in the number of thyroidectomies, the diagnosis of smaller-size nodules due to the use of imaging techniques, and the iodization status of the population are factors that can partially account for our findings. However, further studies are needed to identify other etiological factors related to the increase in the incidence of thyroid cancer.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Rojo Álvaro J, Bermejo Fraile B, Menéndez Torre E, Ardanaz E, Guevara M, Anda Apiñániz E. Aumento de la incidencia de cáncer de tiroides en Navarra. Evolución y características clínicas, 1986–2010. Endocrinol Diabetes Nutr. 2017;64:303–309.