Patients with HIV+ often present lipid disturbances. The role of ghrelin and obestatin in these lipid disturbances is not clear. The effect of antiretroviral (ART) drugs on those molecules is also unknown. This study measured ghrelin and obestatin levels, as well as metabolic markers, in patients with HIV+ before and after 36 weeks of ART.
Material and methodsTwenty HIV-positive, ART-naïve patients who started a scheme consisting of tenofovir/emtricitabine+lopinavir/ritonavir were enrolled. Plasma samples were collected before and after 36 weeks of treatment. Serum ghrelin and obestatin levels were quantitated by ELISA; glucose, cholesterol, and triglyceride levels were measured by colorimetric and enzymatic methods, and cardiovascular risk was calculated by the atherogenic index of plasma (AIP).
ResultsAll patients completed 36 weeks of ART. Total cholesterol (p<0.001), LDL-C (p=0.019), HDL-C (p=0.003), VLDL-C (p=0.002), and triglyceride levels (p=0.021) significantly increased after treatment. AIP revealed increased cardiovascular risk at baseline, which remained high after treatment. There was a statistically significant increase in obestatin level in the unpaired and paired analyses, while ghrelin levels only showed a trend to increase. Changes in ghrelin and obestatin levels positively correlated, but no correlation was seen with any metabolic parameter.
ConclusionAfter 36 weeks of ART, patients showed an altered lipid profile, but there were no significant changes in cardiovascular risk. Ghrelin and obestatin levels increased after 36 weeks of ART, but the increase was only significant for obestatin. Changes in ghrelin and obestatin positively correlate.
Los pacientes con VIH+ frecuentemente presentan alteraciones del perfil lípidico. El papel de ghrelina y obestatina en estas complicaciones no está claro. El efecto del tratamiento antirretroviral (TAR) en dichas moléculas es desconocido. Este estudio determinó los niveles de ghrelina y obestatina, así como los parámetros metabólicos en pacientes VIH+ antes y después de 36 semanas del TAR.
Material y métodosParticiparon 20 pacientes VIH+, vírgenes a TAR, que iniciaron con un esquema de tenofovir/emtricitabina + lopinavir/ritonavir. Se tomaron muestras de plasma antes y después de 36 semanas de tratamiento. Los niveles séricos de ghrelina y obestatina fueron cuantificados por ELISA, los parámetros bioquímicos fueron determinados por métodos colorimétricos, se evaluó el riesgo cardiovascular por medio del índice aterogénico del plasma (AIP).
ResultadosLos pacientes completaron 36 semanas del TAR. Los niveles de colesterol total (p<0,001), c-LDL (p=0,019), c-HDL (p=0,003), c-VLDL (p=0,002) y triglicéridos (p=0,021) mostraron un incremento estadísticamente significativo posterior al tratamiento. El AIP reveló un riesgo cardiovascular alto. Los niveles de obestatina se incrementaron significativamente en el análisis pareado y no pareado; y ghrelina solo mostró tendencia al incremento. Los cambios en ghrelina y obestatina correlacionaron positivamente, sin embargo no correlacionaron con los parámetros metabólicos.
ConclusiónLos pacientes VIH+ mostraron un perfil lipídico alterado después de 36 semanas del TAR. Los niveles de ghrelina y obestatina se incrementaron tras 36 semanas del TAR. El riesgo cardiovascular es persistente. Los cambios en ghrelina y obestatina mostraron una correlación positiva.
Human immunodeficiency virus (HIV) infection affects more than 30 million people worldwide and constitutes an important health concern. The introduction of antiretroviral therapy (ART) has shown great effectiveness in controlling viral replication among HIV infected patients. Nevertheless, ARTs’ use has been associated with a number of important adverse effects that lead to a higher prevalence of cardiovascular risk factors.1 This increased risk is partially related to metabolic disorders, including alterations in lipids and their metabolism.2 Although there are consistent reports of lipid level alterations in ART treated patients,2–4 the mechanism of these alterations is not entirely clear.
Ghrelin and obestatin are hormones coded in the same gene and produced mainly in the stomach and the gastrointestinal tract but with different functions.5,6
Ghrelin is mainly involved on growth hormone (GH) secretion, appetite stimulation and food intake; secondary functions include the promotion of gastric acid secretion, the increase in intestinal motility, regulation of: body weight, glucose and lipid metabolism, and suppression of inflammation. Its function as an orexigenic hormone is independent of the stimulatory effects on GH secretion.6–8 Ghrelin's food intake induction is exerted by the stimulation of hypothalamic neurons that expresses the ghrelin receptor GHS-R9 and it also shows direct adipogenic and lipogenic effects in adipose tissue.10 Further investigations have also demonstrated positive actions of ghrelin on blood pressure and cardiovascular system, involving promotion of diuresis and vasodilatation trough dependent and independent nitric oxide mechanisms and cardiac output by the activity of central nerve system.11,12
Obestatin has been implicated in suppressing food and water intake, decreasing body weight, promoting sleep, and decreasing the contractile force of jejunal muscle fibers.13–15 However, suppression of food intake action is controversial given the fact that recent studies have shown that obestatin putative receptor GPR39, is not expressed in the hypothalamus,13 nonetheless, GPR39 is expressed on adipose tissue16 and obestatin stimulates adipogenesis by the upregulation of adipogenic transcription factors such as PPAR gamma and C/EBP, and by the activation of Akt and further GLUT4 translocation in adipocytes.17 Additionally, obestatin favors the survival of pancreatic beta cells thus increasing insulin secretion.14 Both ghrelin and obestatin have a direct role in the regulation of hunger and satiety, as well as adipogenesis; alterations in their serum concentrations have been associated with very prevalent metabolic diseases such as obesity and DM2.18
It has been shown that ghrelin levels are increased in HIV-infected patients who develop hypertriglyceridemia, suggesting a role for ghrelin on metabolic disorders associated with HIV infection3; nevertheless, the effect of ART has not been controlled and then, its impact on ghrelin or obestatin levels has not been studied so far.
Then, it is not clear if ghrelin and obestatin have any say in the metabolic derangements that affect HIV-infected patients after the use of ART, and it is unknown if ART has any effect on circulating levels of these hormones. Therefore, the aim of this study was to determine the effect of 36 weeks of ART on ghrelin and obestatin circulating levels and its association with metabolic markers and cardiovascular risk in HIV+ patients. This will further develop our understanding of the pathogenesis behind metabolic disorders associated with HIV and their association with ART.
Materials and methodsStudy populationThe study was designed as descriptive, observational, prospective and analytical. For that, 20 HIV+ ART naïve patients were assessed at baseline and prospectively followed for 36 weeks after initiation of ART. We included all patients older than 18 years that were deemed candidates for tenofovir/emtricitabine+lopinavir/ritonavir (FTC/TDF+LPV/r) as initial therapy by an infectious diseases specialist. By using the same ART regime, we aimed at treatment bias avoidance. Patients with any previous ART scheme, known primary resistance to any of the FTC/TDF or LPV/r medication, or active illicit drug use, were not included. The Atherogenic Index of Plasma (AIP) was used to determine cardiovascular risk as described elsewhere.19
The study was approved by the local ethics committee in accordance to the Declaration of Helsinki and all patients provided written informed consent before enrollment.
Biochemical and hormonal analysisBlood samples were drawn after an 8 hour fast at baseline and after 36 weeks of ART. A minimum treatment adherence of 80%, evaluated by direct questioning and remaining pills, was required to be included in the analysis. Concentrations of plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), very low-density lipoprotein cholesterol (VLDL-c), and triglycerides (TG) were obtained by standard biochemical methods (BioSystems®, Barcelona, Spain). Total plasma ghrelin and obestatin levels were measured separately by Enzyme Immunoassay with a detection range of 0.1–1000ng/mL each (RayBiothech, Inc., Norcross GA, USA); intra- and inter-assay coefficients of variation were <10% and <15% respectively, for both assays.
Statistical analysisAll the analyses were performed using GraphPad Prism 6.0 (GraphPad Software®, La Jolla, CA, USA). Comparative analysis consisted of paired and unpaired non-parametric tests. To test for the association of changes on ghrelin or obestatin levels with changes on metabolic parameters Δvalues were calculated according to the formula: Δvalue=Week36 value−Baseline value; then, Spearman's correlation test was performed. A two-tailed p-value <0.05 was considered statistically significant.
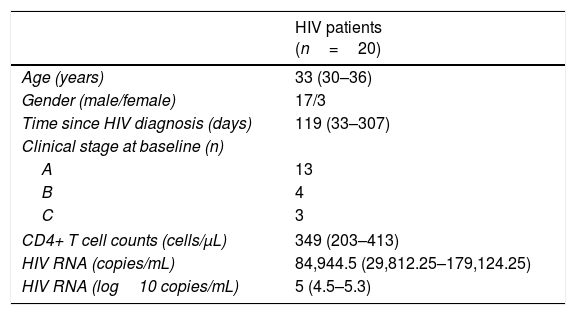
ResultsAll patients completed follow-up assessment at 36 weeks after ART initiation. Baseline demographic and HIV-relevant characteristics are depicted on Table 1. Median age was 33 years and median baseline body mass index (BMI) was 24kg/m2 (interquartile range: 20–24kg/m2); as expected, all patients were on active viral replication at inclusion.
Clinical and demographical characteristics of study subjects.
| HIV patients (n=20) | |
|---|---|
| Age (years) | 33 (30–36) |
| Gender (male/female) | 17/3 |
| Time since HIV diagnosis (days) | 119 (33–307) |
| Clinical stage at baseline (n) | |
| A | 13 |
| B | 4 |
| C | 3 |
| CD4+ T cell counts (cells/μL) | 349 (203–413) |
| HIV RNA (copies/mL) | 84,944.5 (29,812.25–179,124.25) |
| HIV RNA (log10 copies/mL) | 5 (4.5–5.3) |
Data are presented as median (interquartile range).
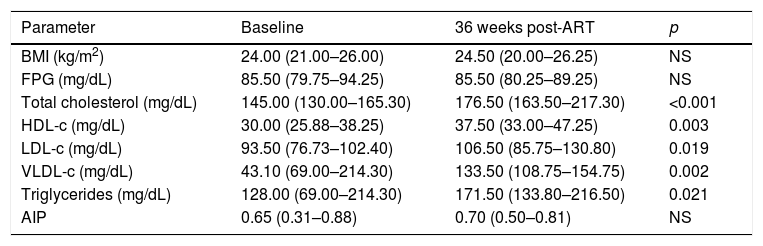
After 36 weeks of ART, all patients achieved undetectable HIV RNA levels (median: 1.6log10copies/mL) and increased their CD4+ T cell counts (median: 544cells/μL). We observed a statistically significant increase of TC (p<0.001), LDL-c (p=0.019), HDL-c (p=0.003), VLDL-c (p=0.002) and TG levels (p=0.021) after ART. However, no changes were detected in BMI and FPG. The baseline median AIP was elevated [0.65 (0.31–0.88)] and no significant differences were perceived after 36 weeks of ART [0.70 (0.50–0.81)] (Table 2).
Metabolic parameters of HIV+ patients before and after 36 weeks of ART.
| Parameter | Baseline | 36 weeks post-ART | p |
|---|---|---|---|
| BMI (kg/m2) | 24.00 (21.00–26.00) | 24.50 (20.00–26.25) | NS |
| FPG (mg/dL) | 85.50 (79.75–94.25) | 85.50 (80.25–89.25) | NS |
| Total cholesterol (mg/dL) | 145.00 (130.00–165.30) | 176.50 (163.50–217.30) | <0.001 |
| HDL-c (mg/dL) | 30.00 (25.88–38.25) | 37.50 (33.00–47.25) | 0.003 |
| LDL-c (mg/dL) | 93.50 (76.73–102.40) | 106.50 (85.75–130.80) | 0.019 |
| VLDL-c (mg/dL) | 43.10 (69.00–214.30) | 133.50 (108.75–154.75) | 0.002 |
| Triglycerides (mg/dL) | 128.00 (69.00–214.30) | 171.50 (133.80–216.50) | 0.021 |
| AIP | 0.65 (0.31–0.88) | 0.70 (0.50–0.81) | NS |
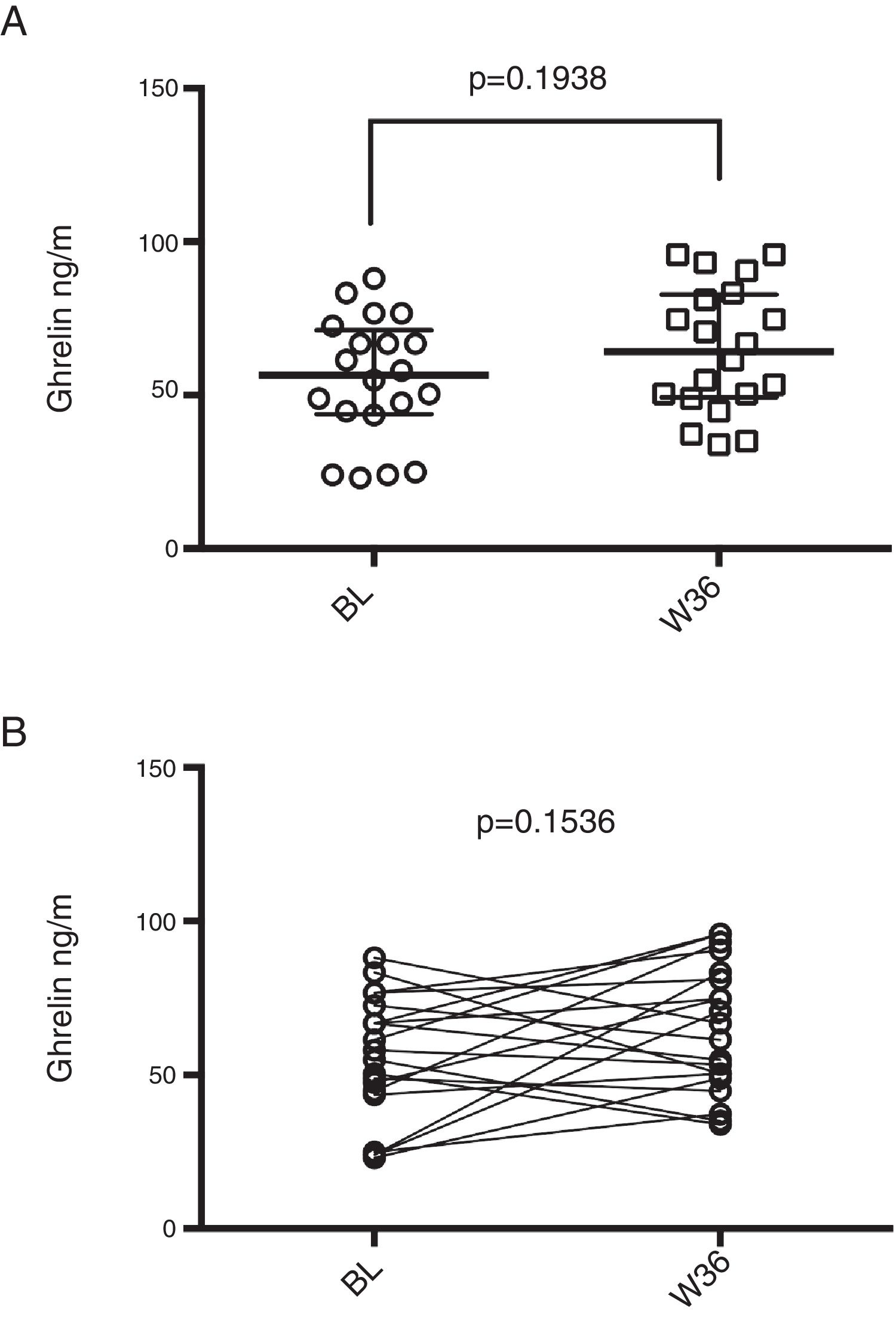
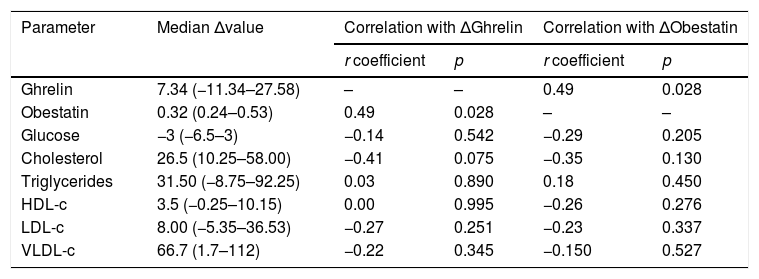
Median baseline ghrelin levels were 56.52ng/ml (43.86–71.17ng/ml) and a slight increase was observed after 36 weeks of ART [64.15 (49.33–82.80) ng/ml], however, it was not statistically significant (p=0.194; Figure 1A). When performing a paired analysis, no significance was also observed (p=0.154; Figure 1B).
Ghrelin levels in HIV infected patients prior and post-36 weeks of ART. (A) Median ghrelin levels of HIV patients before (BL) and after 36 weeks of ART (W36), unpaired comparison was performed by means of Mann–Whitney test. (B) Paired comparison of ghrelin levels before and after 36 weeks of ART, Wilcoxon matched-pairs signed rank test was performed.
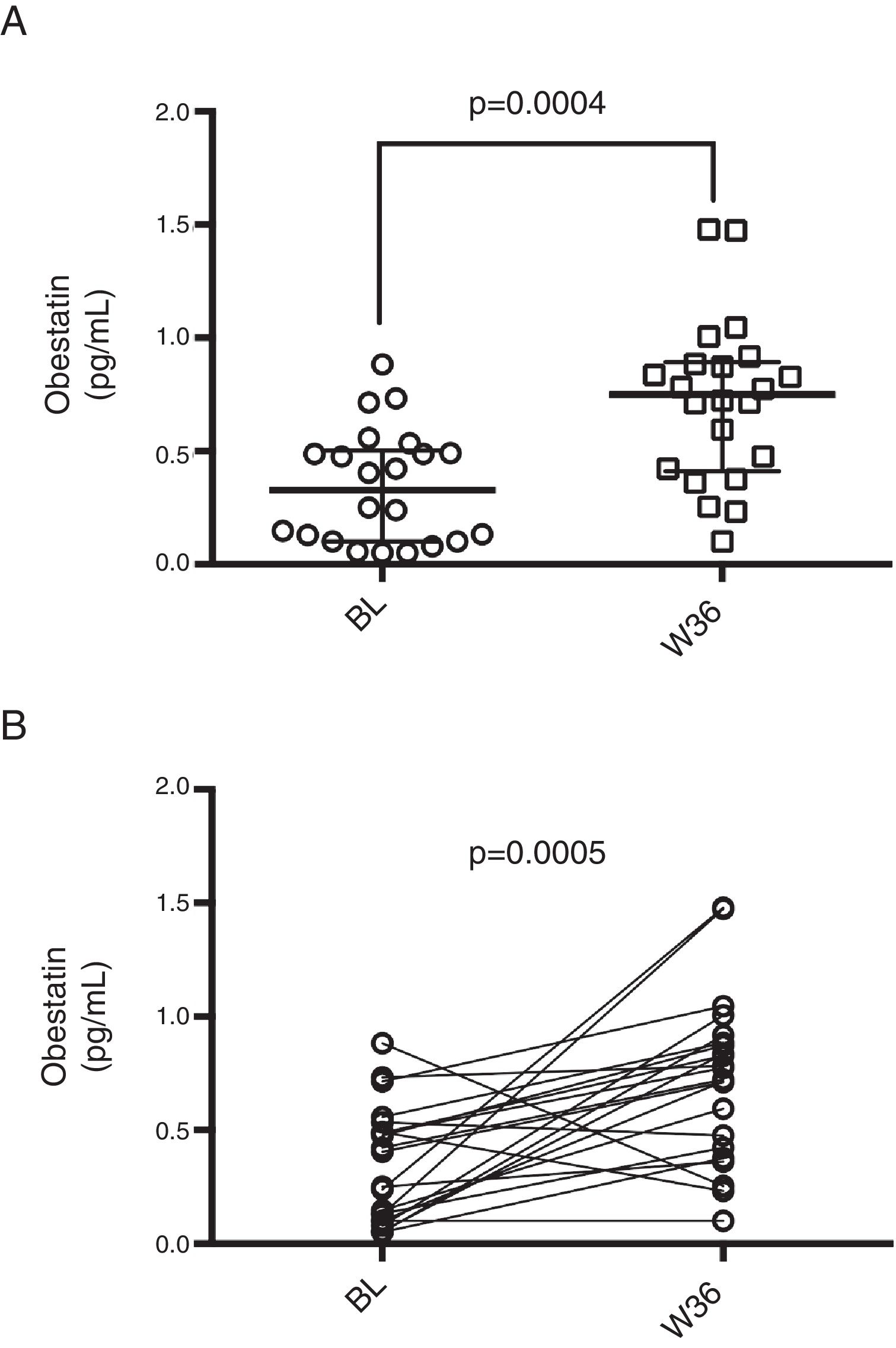
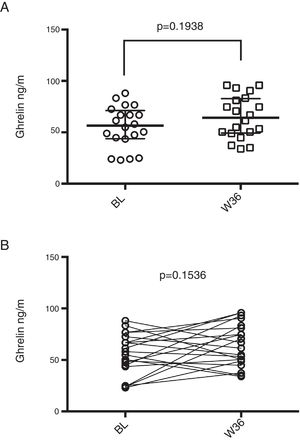
On the other hand, median basal obestatin levels were 0.33pg/mL (0.10–0.50pg/mL) and after 36 weeks of ART, the levels increased to 0.75pg/mL (0.41–0.89pg/mL). Obestatin levels showed a statistically significant increase both in the unpaired and paired analyses (p<0.001 for both; Figure 2).
Obestatin levels in HIV infected patients prior and post-36 weeks of ART. (A) Median obestatin levels of HIV patients before (BL) and after 36 weeks of ART (W36), unpaired comparison was performed by means of Mann–Whitney test. (B) Paired comparison of obestatin levels before and after 36 weeks of ART, Wilcoxon matched-pairs signed rank test was performed.
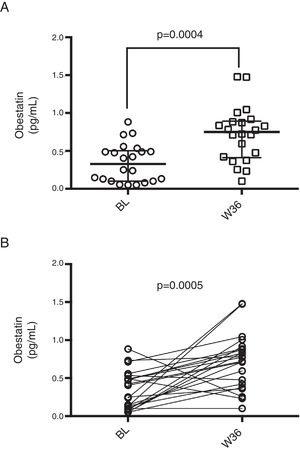
Finally, to test the association of changes in ghrelin or obestatin serum levels with changes in metabolic parameters, we first calculated a delta (Δ) value for each parameter according to the formula: Δvalue=Week36 value−Baseline value. After that, we tested for correlations of obestatin and ghrelin Δvalues with Δvalues of metabolic parameters, results are shown in Table 3. Only a positive correlation was detected for ΔGhrelin and ΔObestatin values which indicates that an increase (or decrease) in ghrelin levels is accompanied by an increase (or decrease) in obestatin levels in the same patient. Nevertheless, no correlation was observed for ΔGhrelin or ΔObestatin and any other metabolic parameter.
Correlation of changes in ghrelin or obestatin and metabolic parameters.
| Parameter | Median Δvalue | Correlation with ΔGhrelin | Correlation with ΔObestatin | ||
|---|---|---|---|---|---|
| r coefficient | p | r coefficient | p | ||
| Ghrelin | 7.34 (−11.34–27.58) | – | – | 0.49 | 0.028 |
| Obestatin | 0.32 (0.24–0.53) | 0.49 | 0.028 | – | – |
| Glucose | −3 (−6.5–3) | −0.14 | 0.542 | −0.29 | 0.205 |
| Cholesterol | 26.5 (10.25–58.00) | −0.41 | 0.075 | −0.35 | 0.130 |
| Triglycerides | 31.50 (−8.75–92.25) | 0.03 | 0.890 | 0.18 | 0.450 |
| HDL-c | 3.5 (−0.25–10.15) | 0.00 | 0.995 | −0.26 | 0.276 |
| LDL-c | 8.00 (−5.35–36.53) | −0.27 | 0.251 | −0.23 | 0.337 |
| VLDL-c | 66.7 (1.7–112) | −0.22 | 0.345 | −0.150 | 0.527 |
Ghrelin and obestatin are peptide hormones with apparently antagonic metabolic actions despite being coded on the same gene.6 HIV-infected patients suffer from a number of metabolic disorders through the course of their illness and, although undeniably efficacious for viral replication control, ART exacerbates those alterations.1 To date, the association between HIV-related metabolic disorders with ghrelin and obestatin and the possible influence that ART may have on these hormones remains elusive. Therefore, we aimed to determine the effect of 36 weeks of ART on ghrelin and obestatin levels and their association with metabolic parameters on HIV patients.
The fact that HIV infected individuals on ART develop dyslipidemia (hypertriglyceridemia and hypercholesterolemia) has been thoroughly demonstrated, especially after the use of protease inhibitors.20 In our study, individuals showed increased levels of cholesterol, triglycerides, HDL-c and LDL-c after 36 weeks of an ART scheme consisting of FTC/TDF+LPV/r. In addition, our data show that even before initiating ART, patients had an increased AIP index, thus a moderate to high cardiovascular risk, even higher than what has been reported for hypertensive or type 2 diabetes mellitus.21
Also, we observed increased levels of both ghrelin and obestatin after ART, although only obestatin levels reached statistical significance. Despite ART associated ghrelin increase, HIV patients showed significantly lower ghrelin levels than lean healthy individuals as previously reported by our group (177.6±72.8pg/mL)22 (a statistical comparison was performed with data and a p value of 0.002 was determined). The same is observed for obestatin, although in this case, the difference on HIV positive and HIV negative values is about three orders of magnitude (unpublished data; p<0.001).
Even though literature is scarce in terms of why and what causes obestatin levels to change, we found an increment after ART, suggesting that some pathway involved in this treatment could favor obestatin production. Also, it has previously been shown that obestatin acts to restrict the development of foam cells formation by preventing low-density lipoproteins oxidation,23 and it could be possible that the ART consequent dyslipidemia in which levels of LDL increases as well set an impulse to secrete more obestatin as a mean to avoid the possible cardiovascular damage.
One study compared ghrelin levels among HIV infected patients with and without hypertriglyceridemia and found that hypertriglyceridemic patients have higher ghrelin levels and that ghrelin and triglyceride levels correlate among these patients,3 suggesting a role for ghrelin on HIV-associated metabolic disorders. Nevertheless, all patients included in this study were under ART for long-term (>1 year), and ART schemes were heterogeneous; then, the role of ghrelin on ART-induced metabolic disorders was not examined.
In agreement to our results, low ghrelin levels have been reported in HIV+ lipodystrophic patients when compared to non-lipodystrophic HIV+ patients.24 This may suggest the presence of a process directly related to HIV infection that negatively affects ghrelin. On the other hand, Freitas et al., reported significantly increased ghrelin levels in HIV infected patients as compared to uninfected subjects25 nevertheless, a heterogeneous group of HIV+ subjects in terms of ART and clinical characteristics of disease was included in that study and this may explain the differences observed. To the best of our knowledge, this is the first study that measures serum obestatin levels on HIV-infected patients and that explores the effect of ART on ghrelin and obestatin levels.
The gastrointestinal tract is a fundamental site of HIV replication. In turn, this leads to HIV enteropathy which is characterized by the chronic inflammation of the intestinal mucosa due to the vast depletion of CD4+ T cells accompanied by a decreased mucosal repair and regeneration.26 It is then possible that ghrelin and obestatin intestinal producing cells are affected in number or function and the reduction in levels observed in HIV patients is a consequence of this effect. The use of ART efficiently suppresses viral replication but is not as efficient in recovering the immune function of patients27; so, a partial recovery of intestinal immune and barrier function after ART may be reflected as an increase on ghrelin and obestatin levels, which nevertheless, remain lower than those of healthy subjects. HIV enteropathy also increases intestinal barrier permeability and microbial translocation, which favors a chronic systemic inflammatory state. Ghrelin protects against chronic intestinal inflammation28 and the decrease of ghrelin levels seen on HIV patients may favor the persistence of HIV enteropathy thus potentially creating a vicious cycle.
It is important to point out that ghrelin exists in two forms: acylated and unacylated ghrelin, and some studies have shown that these two forms have different functions. Acylated ghrelin is a hormone that promotes appetite leading to a positive energy balance and weight gain. This hormone has been proposed as an adaptive mechanism to caloric restriction conditions. On the other hand, unacylated ghrelin was first described as an inactive molecule, nevertheless it has shown to be an appetite inducer, temperature regulator, glucose-dependent insulin secretor, to be active in muscle atrophy, and to be involved in lipid metabolism, favoring lipid storage.6,29 Nevertheless, in this study we only measured total ghrelin, and this must be considered as a limitation.
Other limitations of our study include the lack of other metabolic measures such as insulin or C reactive protein, as well as the restricted sample size. Nonetheless we identify other possible research questions that will better delineate the metabolic alterations seen in HIV patients. For example, a detailed body composition and an evaluation of hunger and satiety in HIV patients with respect to hormone levels and ART would be interesting to perform, as well as it would be to test the association of ghrelin and obestatin with HIV enteropathy and immune parameters of chronic inflammation both in the presence and absence of ART, and maybe in the future, the possible effect of ghrelin or obestatin analogs on such variables.
More studies are needed in order to better understand the role of HIV infection and ART on ghrelin and obestatin levels and their function, as well as their metabolic effect and the immunologic interplay on the context of a chronic infection.
ConclusionsThe use of ART during 36 weeks is associated with an increase in obestatin, but not ghrelin levels in HIV infected subjects.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that there is no conflict of interests regarding the publication of this paper.
The authors thank M.D. Juan Luis Alcalá Zermeño for his advice.