Microscopic colitis is a generic term that includes 2 main forms, collagenous colitis and lymphocytic colitis, and describes a form of inflammatory bowel disease with a chronic and relapsing course. The incidence of microscopic colitis is between 2 and 8 times higher in women than in men, although age, more than sex, increases the risk of collagenous colitis (odds ratio [OR] 8.3 for age ≥65 vs. <65 and OR 2.8 for women). The main symptom is chronic non-bloody watery diarrhoea. Other common symptoms include abdominal pain (50%–70%), with the result that many patients with microscopic colitis meet criteria for irritable bowel syndrome. Colonoscopy with multiple colonic biopsies is currently recommended, as histological changes are the main characteristic feature. The colonic mucosa is macroscopically normal, although certain minimal endoscopic abnormalities have been described.
Colitis microscópica es un término genérico que incluye 2 formas principales, colitis colágena y colitis linfocítica, que describe una forma de enfermedad inflamatoria intestinal con curso crónico y recidivante. La incidencia de colitis microscópica es entre 2 y 8 veces más alta en mujeres que en hombres; sin embargo, la edad contribuye más que el sexo en el riesgo de colitis colágena (OR 8,3 para edad ≥65 vs. <65años y OR 2,8 para sexo femenino). El síntoma principal es la diarrea crónica acuosa, no sanguinolenta. Otros síntomas frecuentes incluyen el dolor abdominal (50-70%), lo que hace que muchos pacientes con colitis microscópica cumplan criterios de síndrome de intestino irritable. Hoy en día se recomienda la realización de una colonoscopia, con toma de biopsias escalonadas en todos estos pacientes, ya que el diagnóstico es principalmente histológico. La mucosa colónica suele ser macroscópicamente normal, aunque se han descrito alteraciones endoscópicas mínimas.
Microscopic colitis (MC) is a generic term that includes two main forms, collagenous colitis (CL) and lymphocytic colitis (LC), and describes a form of inflammatory bowel disease (IBD) characterised by:
- 1.
Chronic or intermittent non-bloody watery diarrhoea.
- 2.
Macroscopically normal or almost normal colonic mucosa assessed by colonoscopy.
- 3.
Characteristic histopathological findings.
In 1993, French and American research groups suggested the use of MC as a generic term to cover any type of colitis in which there were histological, but not endoscopic or radiological, abnormalities. This later became the generic term for the two main entities, known as CC and LC,1 which are characterised clinically by chronic non-bloody watery diarrhoea. Nevertheless, some authors consider that CC and LC should be regarded as histological subtypes of the same disease, and not as different entities,2 a hypothesis that is widely debated.3
There are few studies on the clinical course of patients with MC, but the disease is considered to be characterised by chronic or intermittent diarrhoea and recurrent symptoms, Although the colonic mucosa is macroscopically normal in most patients, it can present mild oedema and erythema. Mucosal tears or fractures and an abnormal vascular pattern have also been described occasionally, especially in patients with CC. MC is characterised by microscopic findings that differ for CC and LC, and which are specific to each entity. These aspects will be discussed in detail in the following sections.
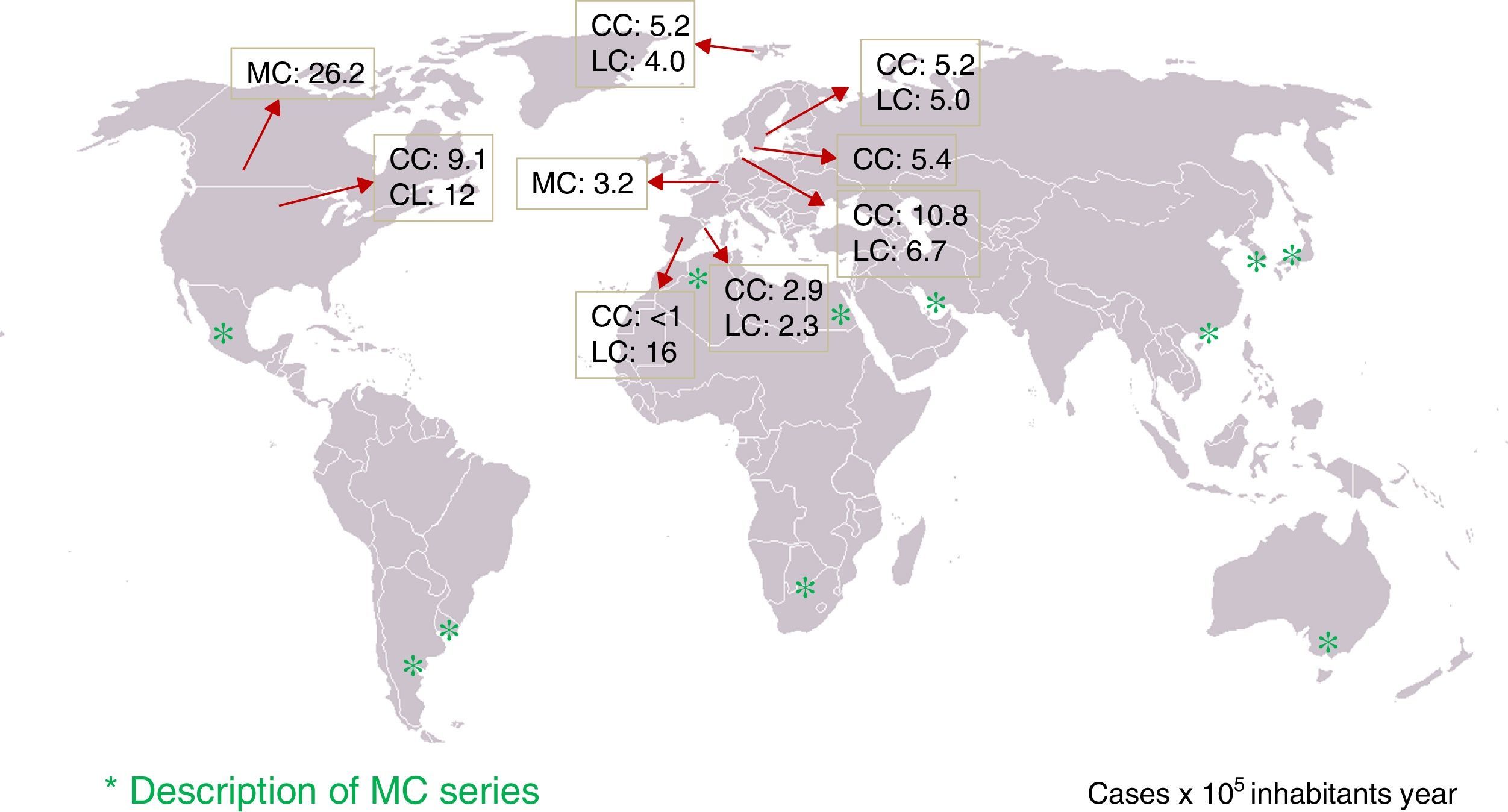
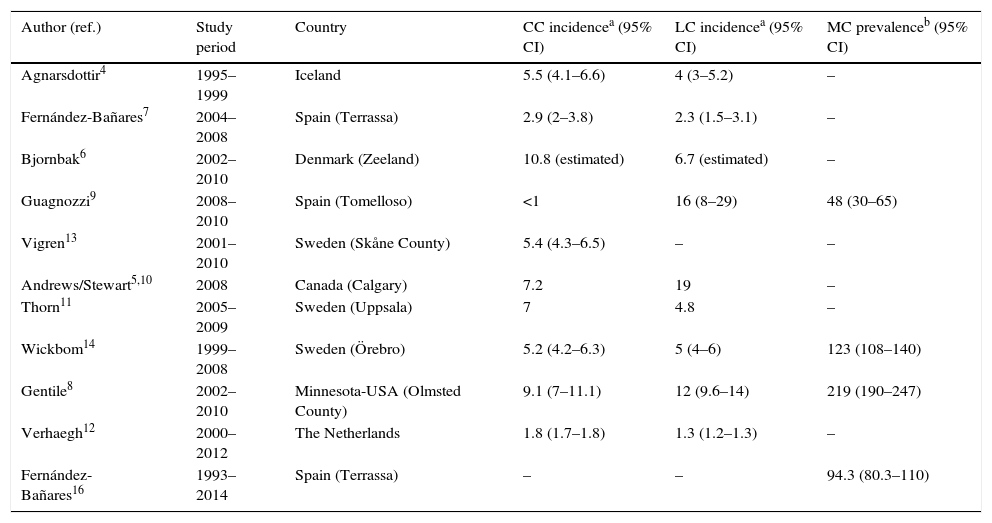
Epidemiology: incidence of microscopic colitis and frequency in patients with chronic diarrhoeaTen population-based studies on the incidence of CC and/or LC in five European countries (Sweden, Iceland, Denmark, the Netherlands and Spain) and two North American countries (United States and Canada) have been published4–14 (Table 1). Four of these studies reported that the incidence had increased over several decades15–19. The incidence of CC in northern Europe and North America varies from 5.2 to 10.8 (×100,000 person-years), and in Spain from <1 to 2.9. Unfortunately, no studies have been conducted in other southern European countries to determine whether there are north–south differences in incidence. The incidence of LC varies from 4 to 19 (×100,000 person-years) in northern Europe and North America, and from 2.3 to 16 in Spain.
Population-based studies on the incidence and prevalence of collagenous colitis and lymphocytic colitis. Only the most recent data in studies assessing the trend in the incidence are provided.7,8,10,14
| Author (ref.) | Study period | Country | CC incidencea (95% CI) | LC incidencea (95% CI) | MC prevalenceb (95% CI) |
|---|---|---|---|---|---|
| Agnarsdottir4 | 1995–1999 | Iceland | 5.5 (4.1–6.6) | 4 (3–5.2) | – |
| Fernández-Bañares7 | 2004–2008 | Spain (Terrassa) | 2.9 (2–3.8) | 2.3 (1.5–3.1) | – |
| Bjornbak6 | 2002–2010 | Denmark (Zeeland) | 10.8 (estimated) | 6.7 (estimated) | – |
| Guagnozzi9 | 2008–2010 | Spain (Tomelloso) | <1 | 16 (8–29) | 48 (30–65) |
| Vigren13 | 2001–2010 | Sweden (Skåne County) | 5.4 (4.3–6.5) | – | – |
| Andrews/Stewart5,10 | 2008 | Canada (Calgary) | 7.2 | 19 | – |
| Thorn11 | 2005–2009 | Sweden (Uppsala) | 7 | 4.8 | – |
| Wickbom14 | 1999–2008 | Sweden (Örebro) | 5.2 (4.2–6.3) | 5 (4–6) | 123 (108–140) |
| Gentile8 | 2002–2010 | Minnesota-USA (Olmsted County) | 9.1 (7–11.1) | 12 (9.6–14) | 219 (190–247) |
| Verhaegh12 | 2000–2012 | The Netherlands | 1.8 (1.7–1.8) | 1.3 (1.2–1.3) | – |
| Fernández-Bañares16 | 1993–2014 | Spain (Terrassa) | – | – | 94.3 (80.3–110) |
Studies that have assessed trends in the incidence of MC have observed an increase in the frequency of both CC and LC in recent decades.7,8,10,14 This could be due to a real increase in incidence as well as a better understanding of the disease.20
Several epidemiological studies have shown that the incidence of MC is between 2 and 8 times higher in women than in men.4,7,14,19,21 Furthermore, MC is a disease that can be diagnosed at any age, although it has been observed more often in elderly patients.4,6–19 It has been suggested that age contributes more than sex to the risk of CC (OR 8.3 for age ≥65 vs. <65 years and OR 2.8 for women),13 and that age ≥65 years increases the risk of developing CC/LC 4.1-fold (95% CI: 3.9–4.4 for CC and 95% CI: 3.8–4.4 for LC).12 Finally, it has been reported that 25% of patients with CC are under 45 years of age at diagnosis,15 and cases of MC have been described in the paediatric population.22–26
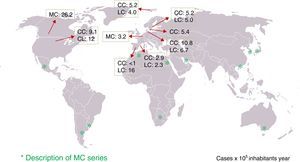
Various studies have also determined the frequency of diagnosis of MC in patients with a history of chronic or intermittent non-bloody watery diarrhoea and normal colonoscopy in whom other causes of these have been excluded, observing MC in 4%–29% of cases.7,9,16–18,27–36 However, the diagnostic protocols prior to colonoscopy with biopsies are not reported in most studies and, therefore, these may not be comparable. Similarly, the causes of chronic diarrhoea may differ according to geographical region. Nevertheless, it is notable that cases of MC have been described in practically all areas of the world (Fig. 1). The frequency of MC in patients with chronic watery diarrhoea increases with age; thus, the frequency of MC in men over 70 or in women over 50 was around 20% in two studies, giving an incidence of MC of 9.5% and 13.7% if the entire age group is considered.9,17
Advances in diagnosisClinical diagnosisClinical symptoms: symptom guideAs mentioned, the main symptom of MC is chronic non-bloody watery diarrhoea, which can be recurrent or intermittent. Other common symptoms include abdominal pain (50%–70%), nocturnal diarrhoea (25%–50%), meteorism, faecal urgency (70%), incontinence (40%), weight loss (up to 50%) and fatigue (50%–60%)37–39. Symptoms can appear months before the diagnosis is made, given their similarity to a functional condition (see below). Forty percent of patients experience acute onset of symptoms.40 There is no specific symptom that discriminates between LC and CC, so characterisation is purely histological.
Clinical courseThe clinical course of MC is benign, with intermittent episodes of diarrhoea in most cases. Only 10%–15% of patients present chronic persistent daily diarrhoea, although this has been determined from studies with a short follow-up or small sample size.41–43 It has been suggested that LC, compared to CC, has a milder course with a greater tendency to spontaneous remission.44 Although MC is a benign disease, rare cases of colonic perforation have been reported, both spontaneous and following colonoscopy, which may be related with the presence of mucosal tears or fractures that can be observed during the colonoscopy (see below).45–48 However, MC does not present a higher risk of colorectal cancer, so no specific screening is required in these patients.35,49–51
MC is associated with certain autoimmune diseases in up to 50% of patients.38 The most common comorbidity in MC is coeliac disease, present in up to 20% of patients, and up to 50 times more likely in patients with MC than in the general population.11 This should be taken into account in patients with MC and inadequate response to specific treatment. Other diseases that are more prevalent in MC than in the general population are type 1 diabetes mellitus, autoimmune thyroiditis, rheumatoid arthritis, Sjögren syndrome, Raynaud syndrome and psoriasis.52
Endoscopic diagnosisIn MC, the appearance of the colon is normal, as its name suggests, but slight changes can occasionally be observed in the mucosa, such as erythema, oedema or an abnormal vascular pattern. Diagnosis cannot be made on the basis of the endoscopic findings, although mucosal abnormalities typical of this entity are increasingly being described.
Patients with non-bloody watery diarrhoea should ideally undergo complete colonoscopy with terminal ileum intubation. If the examination is normal, multiple biopsies should be taken from different segments of the colon. Although some studies have shown that most patients with MC can be identified from distal colon samples,27 others have demonstrated that histological changes may be patchy, so such restrictive sampling could fail to diagnose patients with abnormalities predominantly in the proximal colon.53–55 The changes detected—especially in CC—are usually more severe in the right colon, and both forms of MC may overlap. Likewise, the normal histology of the ascending colon is different from the descending colon.56 All this supports the diagnostic strategy of taking biopsies from different segments of the colon, and labelling the samples separately.37 A minimum of 8 biopsies should be taken along the colon (at least 4 from the proximal colon and another 4 from the descending sigmoid colon).57–59
Follow-up endoscopies are not recommended in MC, and are only indicated in order to exclude other causes of chronic diarrhoea in patients with refractory disease.60
Endoscopic findingsDespite being a common cause of chronic diarrhoea, few authors have discussed the macroscopic findings that may be encountered in the colonoscopy. Some studies published in recent years have shown abnormalities that are characteristic or pathognomonic of MC, especially CC. Findings typical of CC are an abnormal vascular pattern, mucosal nodularity and a sequence of changes ranging from mucosal defects to cicatricial lesions. A recent systematic review proposed classifying these lesions to homogenise their description in future studies.61 Four categories have been described:
- 1.
Pseudomembranes.
- 2.
Variable mucosal vascular pattern alterations ranging from pruning of the mucosal vasculature to a crowded, dilated and tortuous capillary network.
- 3.
Mucosal abnormalities: red spots, mucosal nodularity, textural alterations, which may be evident with or without chromoendoscopy. The mosaic pattern or mucosal nodularity (honeycomb mucosa) has an OR of 19.4, with specificity >99% for the diagnosis of CC.
- 4.
Mucosal defects:
- -
Superficial or deep longitudinal mucosal lacerations (cat scratch colon), which are fresh/haemorrhagic.
- -
Mucosal fractures (deep with occasional exposure of the muscularis mucosae).
Mucosal defects in MC present sharply demarcated margins that allow these lesions to be differentiated from ischaemic colitis. These defects are more commonly seen in the ascending colon as a result of an insult, such as instrumentation or air insufflation, due to the presence of a thick dysfunctional layer of type iii collagen associated with an increased colon diameter in this segment. These findings are non-specific for CC, and have been described in the normal colon (attributed to barotrauma from excessive insufflation), in diversion colitis and even in patients with chronic cholestasis. When these lesions are observed, the risk of perforation during colonoscopy is higher, particularly in the ascending colon.47,48
- -
Mucosal cicatricial lesions, which have been observed in both the index colonoscopy and at follow-up. Both hypertrophic scars and fine lines have been described.
The actual prevalence of the abnormalities seen on the endoscopy is unknown, and is likely higher than the 1% estimated prevalence of major lesions. Oedema, erythema and abnormal vascular pattern have a reported prevalence of up to 30%. The presence of linear ulcers in the colon has been independently associated with the use of non-steroidal anti-inflammatory drugs (NSAID) and the thickness of the collagen band; they have also been reported more often in patients with a history of lansoprazole use.62
Although macroscopic lesions are more common in CC, in a recent study of 14 cases of LC the authors found macroscopic lesions in 7 patients (hypervascularity in 6 patients, exudative bleeding in 3 patients and 1 patient with mucosal oedema and loss of normal vascularity). Furthermore, patients with endoscopic abnormalities had more severe diarrhoea and a history of treatment with acetylsalicylic acid (ASA) or proton pump inhibitors (PPI).63
Given that abnormalities are either absent or barely visible in most cases, endoscopists must actively look for changes in the mucosa to target their biopsies. These abnormalities can be visualised using new high-definition colonoscopes, with sharper images and zoom capabilities.64 There is little evidence to show that the use of indigo carmine chromoendoscopy to identify mucosal changes in MC improves direct vision. Indigo carmine staining has been used in some cases to improve the identification of lesions, and seems useful in the context of subtle changes in the mucosa, or in visualising an abnormal vascular pattern. This technique can show a mosaic pattern in LC and a nodular pattern with grooves in CC, and could therefore hypothetically increase endoscopic diagnostic yield by facilitating targeted biopsies.65,66 However, its usefulness seems limited at present, since it lengthens examination time and few endoscopists are experienced in its use.
Endomicroscopy is a technique that combines normal white-light endoscopy with confocal microscopy, allowing microscopic examination of the epithelial surface and facilitating in vivo diagnosis of some diseases. It has been used in the diagnosis of Barrett oesophagus, coeliac disease, IBD and also in MC. In MC, it allows the collagen band to be located and measured, so that targeted biopsies can be taken67,68; in LC, the increased lymphocytic infiltrate in the lamina propria can be observed. The role of these techniques in the future is uncertain, since their use is not widespread and is currently limited to research.
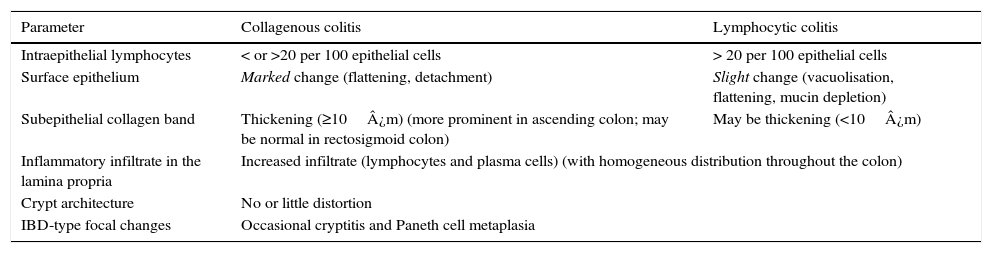
Histological diagnosisCC and LC are two clinicopathological entities defined by histological criteria and basically characterised by the presence of lesions in the stromal–epithelial interface. The morphological criteria for the diagnosis of the two forms of MC are discussed below and summarised in Table 2.1,16,38,60,69–75
Key histological findings in collagenous colitis and lymphocytic colitis.87
| Parameter | Collagenous colitis | Lymphocytic colitis |
|---|---|---|
| Intraepithelial lymphocytes | < or >20 per 100 epithelial cells | > 20 per 100 epithelial cells |
| Surface epithelium | Marked change (flattening, detachment) | Slight change (vacuolisation, flattening, mucin depletion) |
| Subepithelial collagen band | Thickening (≥10¿m) (more prominent in ascending colon; may be normal in rectosigmoid colon) | May be thickening (<10¿m) |
| Inflammatory infiltrate in the lamina propria | Increased infiltrate (lymphocytes and plasma cells) (with homogeneous distribution throughout the colon) | |
| Crypt architecture | No or little distortion | |
| IBD-type focal changes | Occasional cryptitis and Paneth cell metaplasia | |
IBD: inflammatory bowel disease.
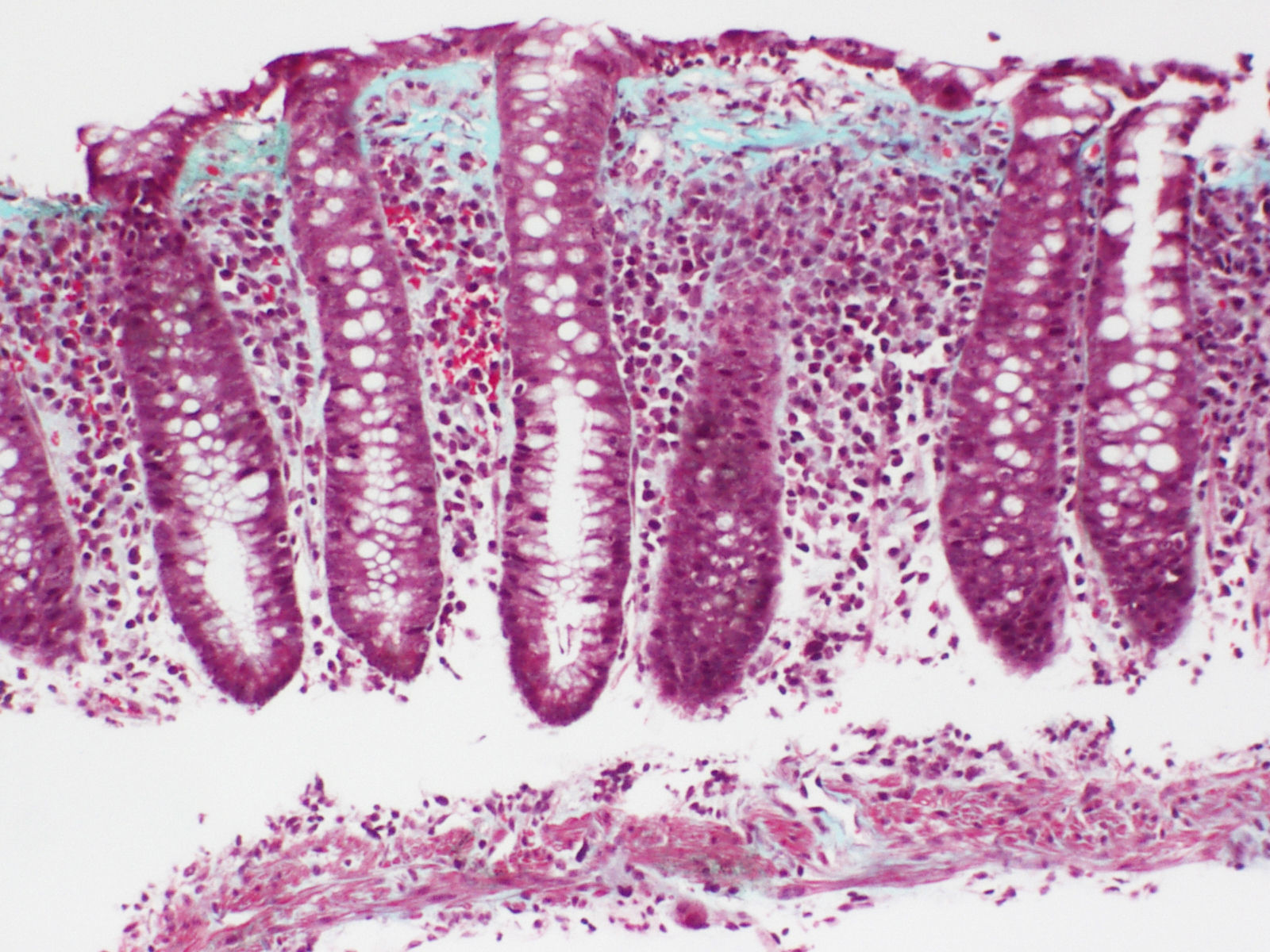
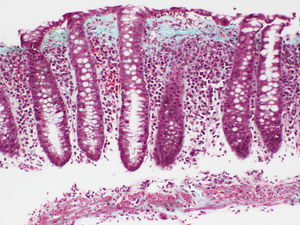
The essential histological finding for diagnosis is an increase in intraepithelial lymphocytes. Most authors establish a minimum of 20 lymphocytes per 100 epithelial cells (normal <5%), although this figure is amply exceeded in most cases. This value has been recently adopted in the European consensus on the histopathology of inflammatory bowel disease.1 Using haematoxylin-eosin staining, the intraepithelial lymphocytes are characterised by a round nucleus with dense chromatin and a clear perinuclear halo (Fig. 2).
The lymphocyte count should be performed in well-oriented sections and in spaces between crypts, without assessing the epithelium of the crypt opening.1,37 In both CC and in LC, intraepithelial lymphocytes are CD8+ T cells which express the usual peripheral T lymphocyte receptors, showing the same immunophenotype as the intraepithelial lymphocytes of the normal mucosa.76 Therefore, although not usually necessary, immunohistochemical staining for CD3—a generic T lymphocyte marker—may be useful for facilitating intraepithelial lymphocyte count in MC.
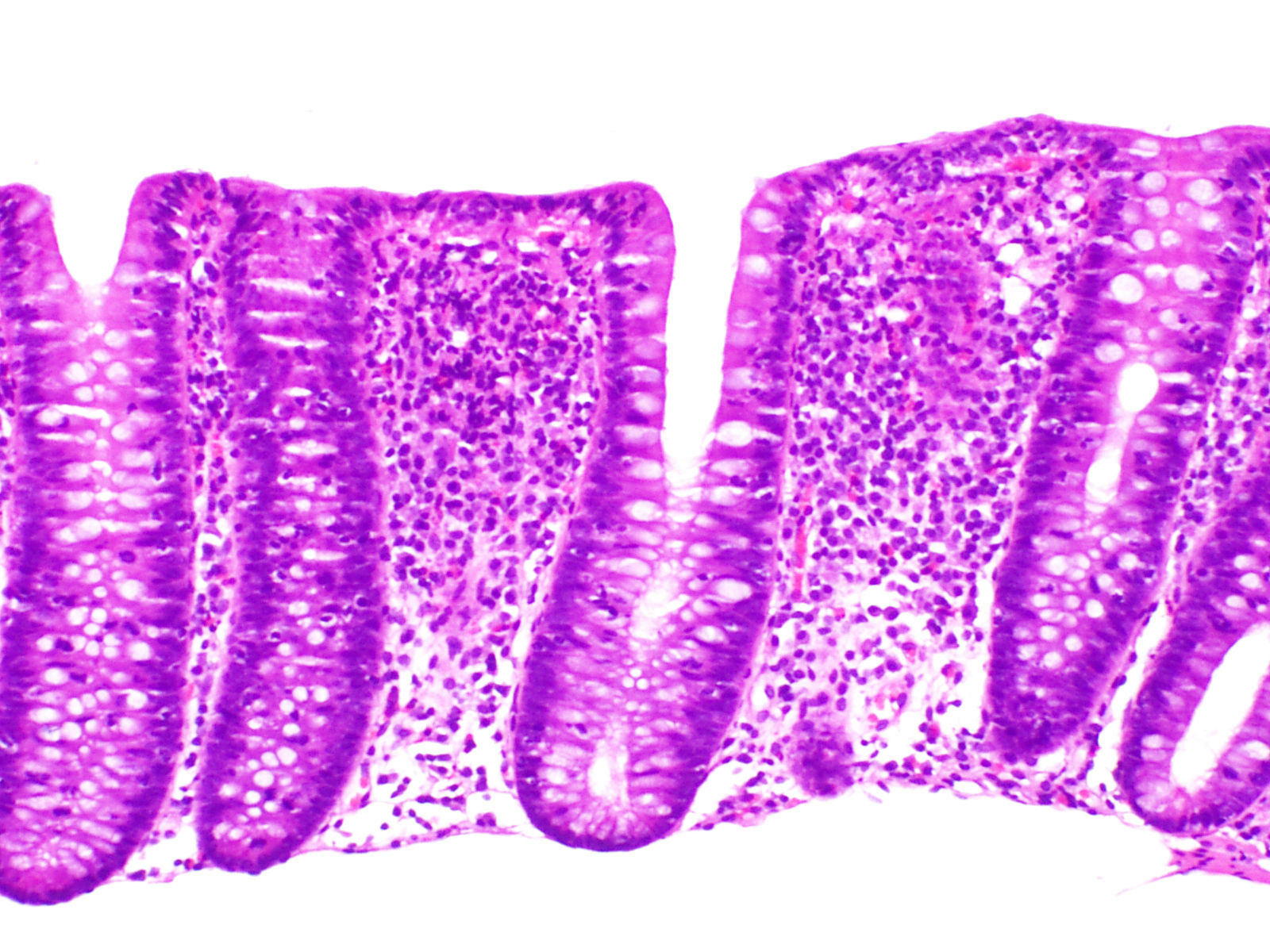
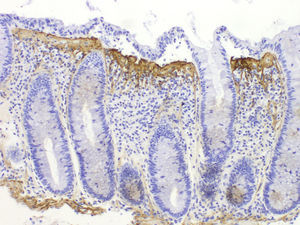
Collagenous colitisAccording to the European Consensus on the Histopathology of Inflammatory Bowel Disease, the essential histological finding for diagnosis is the presence of an irregular collagen deposit, forming a band immediately below the surface epithelium of the mucosa, underlying the basement membrane, with a thickness greater than or equal to 10¿m (normal <3¿m)1 (Fig. 3).
It generally exceeds 10¿m, and in some cases can reach a thickness of more than 50¿m. It usually does not extend around the crypts and has an irregular, frayed appearance at the lower edge; it can contain trapped capillaries, blood cells and inflammatory cells, unlike the clear straight edge of the basement membrane in the normal colon. This qualitative alteration in the subepithelial collagen deposit has been considered by some authors to be more important for diagnosis than the thickness of the band itself.69 In most cases, the diagnosis can be easily established with haematoxylin-eosin staining. In borderline cases, additional collagen stains (e.g. trichrome stains) or tenascin staining can be of great help in the diagnosis.
Using immunohistochemical techniques, this band contains type i, iii and vi collagen, as well as tenascin. Stains for basement membrane components, such as type iv collagen and laminin, do not show staining, confirming that this is not a thick basement membrane, but an abnormal deposit below the basement membrane.
It is very important to take into account that band thickness can only be measured in well-oriented sections cut perpendicular to the mucosal surface (containing crypts sectioned lengthwise), since in poorly-oriented sections, a tangential section of the basement membrane can be erroneously interpreted as thickening.69 The thickness is variable and its distribution is not homogeneous throughout the colon in the same patient; it is generally more evident in the ascending colon, and can decrease or become absent in the distal portions.55,77,78 In this situation, multiple step biopsies of the colon are needed to rule out CC. This heterogeneity in the distribution of the lesions in CC, predominantly in the ascending colon, is not seen in LC, in which the inflammatory changes are generally uniformly distributed throughout the colon.1,16,38,70
Likewise, an increased number of intraepithelial lymphocytes can be observed, although not as high as in LC, and it is not essential for diagnosis.
Common histological aspectsIntraepithelial lymphocyte levels usually increase by over 20% in CC, similar to LC.73,79 Some authors consider that, if all the other criteria are met, CC can be diagnosed even with intraepithelial lymphocyte counts of less than 10%.73
There is a diffuse increase in the cellularity of the lamina propria. The inflammatory infiltrate is composed mostly of lymphocytes, plasma cells and eosinophils. Some authors have reported finding more eosinophils in CC than in LC,69,79 but as this varies greatly, a higher or lower mucosal eosinophil level is not a useful criterion for differential diagnosis. The presence of some neutrophils does not rule out CC, but IBD must be considered when glandular atrophy or distortion and neutrophil-mediated crypt epithelium injury are observed, even in the absence of endoscopic and radiological findings.
The surface epithelium shows degenerative and/or regenerative changes, such as vacuolisation, flattening and loss of mucin. Damage to the surface epithelium, required for the diagnosis of both entities, can vary in severity in different patients and in different areas of the colon in the same patient. Epithelial damage is usually more diffuse in LC; however, epithelial detachment is more common in CC, and it is not unusual to find some samples in which the surface epithelium is completely denuded, leaving the collagen band exposed.69–71
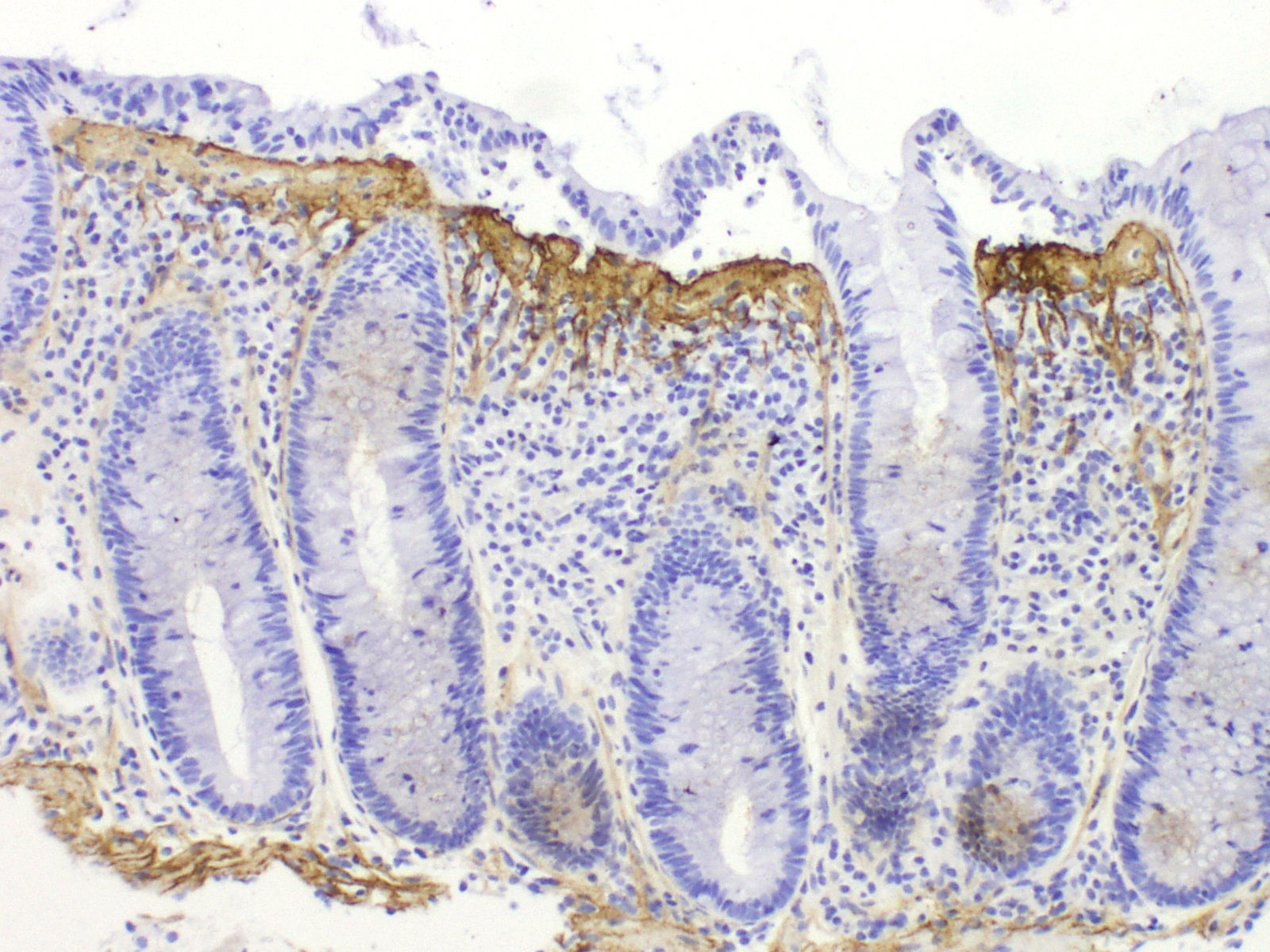
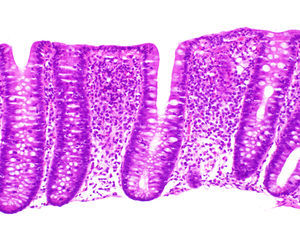
Differential histological diagnosisThe subepithelial collagen band, while necessary for diagnosis, is not sufficient in itself, as thickening of the subepithelial collagen layer can be observed in other conditions, such as megacolon, diverticulitis, hyperplastic polyps or non-neoplastic mucosa in patients with colon cancer. The presence of the collagen band together with other chronic inflammation criteria and an appropriate clinical context are therefore essential for diagnosis. Tenascin immunostaining may be useful in inconclusive cases37,80 (Fig. 4). It has been suggested that the selective accumulation of tenascin in the subepithelial zone is observed exclusively in CC, while other forms of colitis may involve tenascin deposits in the intercryptal matrix.81 The only entity that presented subepithelial tenascin deposits at the limit of normal or somewhat thickened was ischaemic colitis, but unlike CC, these cases also presented deposits in the intercryptal zone (lamina propria fibrosis).
Congo red staining should always be used to be able to properly assess the subepithelial band and to rule out subepithelial accumulation of amyloid substance, which in routine staining has an appearance similar to that of the CC band.
In MC—both CC and LC—there may be active inflammation in the crypts with occasional crypt abscesses, albeit focal and mild and not predominant in the inflammatory infiltrate. The clinical and endoscopic context is of great help in differentiating MC from IBD.
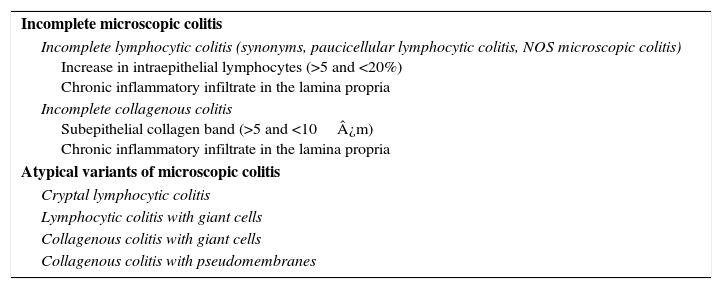
Incomplete and atypical forms of microscopic colitisThe term “incomplete MC” (MCi) has been used to describe those patients with a clinical presentation similar to classic MC but who does not meet the histological criteria for CC or LC (Table 3). This concept is new and controversial. Studies are currently underway to re-evaluate this entity and the response to treatment.
Incomplete and atypical variants of microscopic colitis.
| Incomplete microscopic colitis |
| Incomplete lymphocytic colitis (synonyms, paucicellular lymphocytic colitis, NOS microscopic colitis) Increase in intraepithelial lymphocytes (>5 and <20%) Chronic inflammatory infiltrate in the lamina propria |
| Incomplete collagenous colitis Subepithelial collagen band (>5 and <10¿m) Chronic inflammatory infiltrate in the lamina propria |
| Atypical variants of microscopic colitis |
| Cryptal lymphocytic colitis |
| Lymphocytic colitis with giant cells |
| Collagenous colitis with giant cells |
| Collagenous colitis with pseudomembranes |
New entities, known as atypical forms of MC, have been described in recent years82 (Table 3). Many of them are variants of the classic forms of CC and LC, such as the cryptal variant of LC83 with lymphocytic exocytosis in the crypts and not in the surface epithelium, pseudomembranous CC84,85 and CC with giant cells.86 It should be noted that these forms are extremely rare and they are highly unlikely to constitute specific entities.
Inter- and intraobserver variability in histological criteriaThe usefulness of haematoxylin–eosin diagnostic criteria in MC were evaluated in two studies that measured intra- and interobserver variability. One of these analysed interobserver variability in 90 patient biopsies (20 CC, 20 LC, 20 IBD and 30 normal). Four gastrointestinal pathologists blindly and independently classified the 90 biopsies as CC, LC, chronic colitis, focal active colitis, normal mucosa or other. Interobserver agreement for the 6 categories was acceptable (k=0.68–0.78). Considering the categories of MC versus the other types of colitis, the degree of agreement was very good (k=0.80–0.95). Intraobserver agreement was also good for the 6 categories (k=0.75–0.79), and better at differentiating MC from non-MC (k=0.84–0.91).87 The other study included biopsies from 125 patients diagnosed with CC, LC, MCi, non-specific colitis/IBD and normal biopsies. Interobserver agreement in differentiating MC (CC, LC and MCi) from non-specific forms/IBD and normal biopsies among 3 pathologists with different levels of experience was good (k=0.81–0.89). Interobserver agreement in the differentiation between CC, LC and MCi was lower (k=0.60–0.75), mainly due to less agreement between the evaluations in patients with MCi.88
Diagnosis with biological markersFaecal markers have been shown to be useful for detecting organic disease in the study of patients with chronic diarrhoea, since they accurately determine the presence of inflammation.89,90 Based on this premise, several studies have assessed the benefit of using faecal markers for MC screening.89–91 The most widely used marker is faecal calprotectin, although studies in this marker have included only very small patient numbers, and use different methods and cut-off points, which is a limitation of this marker in general. Furthermore, the findings are contradictory. Thus, while in some studies, 60%–75% of patients with active CC show high faecal calprotectin levels,89,92–94 another study found no differences between active CC and a control group with chronic watery diarrhoea.91
In view of these discouraging results, other more promising faecal markers have been studied. In this respect, it has been suggested that faecal eosinophil cationic protein (ECP) could discriminate clinical activity better than faecal calprotectin.93 Tryptase, eosinophil protein X and myeloperoxidase could also differentiate MC (especially CC) from irritable bowel syndrome (IBS).95 However, larger, prospective studies that include LC are needed to add faecal markers to the routine study of chronic diarrhoea when there is clinical suspicion of MC. Another important point that has yet to be demonstrated is the usefulness of faecal markers in the follow-up of these patients.
Differential diagnosis in patients with chronic non-bloody watery diarrhoeaChronic non-bloody watery diarrhoea is the main symptom of MC which, as mentioned above, can be associated with abdominal pain in up to 50%–70% of patients. These clinical symptoms may be indistinguishable from those presented by patients with diarrhoea-predominant IBS (IBS-D).37–39 In fact, 38%–58% of patients with MC may meet criteria for IBS.96 Patients with IBS-D rarely present nocturnal diarrhoea, and this may be useful in distinguishing between these entities. In any event, diarrhoea due to functional disorders (IBS-D and functional diarrhoea) is up to 100 times more common than diarrhoea secondary to MC.97 This means that the diagnosis of MC should be based on histological and not only clinical parameters. Colonoscopy with biopsies should be mandatory in all patients presenting IBS-D or functional diarrhoea in order to rule out the presence of MC, for which there is effective treatment.91 It is for this reason that attempts have been made to find clinical factors associated with MC that can be used as predictive factors to justify a complete study with colonoscopy and biopsies. To that end, two studies were conducted: a French prospective multicentre study98 and a UK retrospective study.99 Both studies suggest that age >50 years, weight loss and the recent introduction of new medication (mainly PPIs and drugs for the treatment of Parkinson's disease) are associated with a higher risk of MC. The French prospective multicentre study also added the presence of nocturnal diarrhoea and autoimmune comorbidities.98 The authors of the UK study developed a diagnostic score in order to reduce unnecessary costs in patients with a low risk of MC.99 However, the usefulness of the score is limited as it is based on the findings of a retrospective study and has not been confirmed in prospective cohorts.100 Despite this, if clinical suspicion of MC is high due to the presence of the aforementioned risk factors, or there is inadequate response to symptomatic treatment of a condition classified as IBS-D, colonoscopy with step biopsies would be justified.
ConclusionsMC is a common disease in elderly patients, and is underdiagnosed in our setting. The main symptom of MC is chronic, non-bloody watery diarrhoea, which is often associated with a major alteration in the patient's quality of life. Today, MC is diagnosed histologically from biopsies of the colonic mucosa. For this reason, it is essential to perform colonoscopy to the caecum with step biopsies in all patients with chronic watery diarrhoea, even if they have characteristics that suggest functional diarrhoea.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Zabana Y, Ferrer C, Aceituno M, Salas A, Fernández-Bañares F. Colitis microscópica: avances para una mejor identificación en los pacientes con diarrea crónica. Gastroenterol Hepatol. 2017;40:107–116.