Although tumour necrosis factor (TNF) inhibitor drugs are safe and effective, there have been reports of various haematological abnormalities, such as neutropenia, which may lead to serious infections.
We report the case of a 30-year-old woman diagnosed with Crohn’s disease at age 8 with a stenosing pattern and a location in the ileum, colon and small bowel, having previous undergone surgery, on treatment with Humira® [adalimumab (ADA)] 40 mg every 15 days since 2007, which was intensified in 2014 due to loss of response.
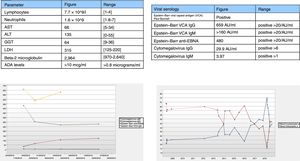
In November 2017, the patient’s treatment was de-intensified owing to persistent clinical remission, and she underwent laboratory monitoring after 4 months with the results shown in (Fig. 1A). Lymphocyte morphology was abnormal with a predominance of large granular lymphocytes (LGLs). The patient’s laboratory testing was extended with viral serology testing (Fig. 1B) and an abdominal ultrasound which identified splenomegaly.
As a myelodysplastic syndrome was suspected, flow cytometry was performed and found no clonal abnormalities suggestive of malignancy.
The patient’s condition was interpreted as secondary to ADA and subsequent viral reactivation. A decision was made to suspend the drug and subsequently monitor the patient with follow-up laboratory testing after two months (Fig. 1C-D).
The patient presented clinical activity 3 months after suspending the medication. Ustekinumab was started, and there have been no subsequent laboratory abnormalities to date (week 52).
Even though TNF inhibitor drugs are safe, owing to their action, a higher risk of infections and haematological disorders such as neutropenia has been reported.1
The drop in neutrophils puts patients at risk of infections and may be due to primary causes (haematological malignancies) or secondary causes, most of which are drug-related toxicities.
TNF inhibitor drugs themselves and secondary morphological abnormalities in lymphocytes, such as LGLs, play a role in neutropenia associated with TNF inhibitor drugs and can suppress haematopoiesis, which may be part of an initial myelodysplastic syndrome.1–4
LGLs comprise a subgroup of T-lymphocytes within natural killer cells which appear normally in 10%–15% of peripheral blood.2,3
They may be of monoclonal origin as in LGL leukaemia, myelodysplastic syndromes, or of non-monoclonal origin secondary to viral infections, haematological disorders such as lymphoma or autoimmune disorders.
Studies from clinical trials in inflammatory bowel disease (IBD) and rheumatoid arthritis have established an incidence of neutropenia (count <1000) of 0.6%–0.9% for ADA and 1.1%–5.7% for infliximab. Despite this, 81% of patients stayed on the same treatment whereas all others switched to TNF inhibitor drugs; however, 62.5% had to suspend them due to recurrent neutropenia.1
In severe neutropenia (count <500), it is recommended that the drug be suspended and that secondary causes such as haematological disorders be investigated.
With these data, transient or persistent neutropenia associated with TNF inhibitor drugs is known to be underestimated with reports only in the form of case series.
Owing to the immunosuppressed conditions associated with TNF inhibitor drugs, such as neutropenia and LGLs, the risk of infections (both primary infections and reactivations), especially viral infections such as Epstein–Barr virus (EBV) infection may also induce changes in B- and T-lymphocyte cell differentiation, which may precipitate persistent myelodysplastic syndromes.1,5
Therefore, there are two concomitant phenomena inducing both neutropenia and LGLs. Our case featured both phenomena (ADA and suspected viral reactivation) as well as lymphocyte abnormalities. The question of the root cause warrants some reflection. It is recognised that EBV reactivation can induce changes in cytomegalovirus (CMV) serology results, as in our patient, and also that viral reactivations in immunosuppressed patients may have few symptoms.4,5 Viral loads were negative on laboratory testing, whereas serological changes persisted, probably because the event was asymptomatic.
Following suspension of the medication, the patient’s peripheral blood morphology returned to normal within a few days, whereas her serological abnormalities remained abnormal; hence, it appears that the presence of LGLs was due to the TNF inhibitor drug and this led to a viral reactivation.
In summary, both TNF inhibitor drugs causing changes in the bone marrow, such as LGLs, and the risk of reactivation of infections, mainly EBV infection, may induce associated myelodysplastic syndromes.
For IBD, this risk has yet to be evaluated, since monitoring guidelines do not include regular serology testing and most studies and case series reported have been in patients with rheumatic diseases.
Please cite this article as: Saldaña Dueñas C, Rodríguez Gutiérrez C. Neutropenia por linfocitos grandes granulares secundaria a adalimumab y reactivación de mononucleosis: reporte de un caso y revisión de la literatura. Gastroenterol Hepatol. 2020;43:446–447.