Hepatic encephalopathy (HE) is a frequent and serious complication of liver cirrhosis.
In addition to correction of the precipitating factors, the most commonly used treatments are non-absorbable disaccharides and rifaximin. Many of the recommendations are based on current clinical practice and there are few randomised controlled trials.
Currently, rifaximin should be initiated during an episode of EH if, after 24–48 hours of non-absorbable disaccharide therapy, there is no clinical improvement. In recurrent EH, it is advisable to add rifaximin in patients under non-absorbable disaccharide therapy who develop a new episode. Currently, standard treatment with rifaximin for minimal EH is not recommended.
Rifaximin is effective in the acute treatment of overt encephalopathy and in preventing recurrence.
La encefalopatía hepática (EH) es una complicación grave y frecuente de la cirrosis hepática.
Además de corregir los factores precipitantes, los tratamientos más utilizados y con los que existe mayor experiencia son los disacáridos no absorbibles y la rifaximina. Muchas de las recomendaciones se basan en la práctica clínica con escasos estudios controlados y aleatorizados.
Actualmente, la rifaximina se debería iniciar durante un episodio de EH, si tras 24–48 horas con tratamiento con disacáridos no absorbibles no presenta mejoría clínica. En la EH recurrente es aconsejable añadir rifaximina si, el paciente estando en tratamiento con disacáridos no absorbibles, presenta clínica. Por el momento, no se recomienda el tratamiento habitual de la EH mínima con rifaximina.
La rifaximina ha demostrado que es eficaz en el tratamiento agudo de la EH así como en la prevención de recidivas.
Hepatic encephalopathy (HE) is a serious and common complication of liver failure, occurring in 30–45% of patients with cirrhosis1 and in 10–50% of patients with transjugular intrahepatic portosystemic shunt (TIPS).2
HE is characterised by a wide spectrum of neuropsychiatric and motor abnormalities that can cause symptoms such as slight impairment in cognitive and motor function, or even coma and death.3,4 It is one of the most debilitating manifestations of liver disease, and severely affects the quality of life of patients and their carers. It also results in the utilisation of more resources than other manifestations of cirrhosis.2,5
The onset of HE is an indicator of poor prognosis, and it has been shown to be an independent predictor of mortality: the probability of 1- and 3-year transplant-free survival after the first episode of acute HE is approximately 50% and 25%, respectively.1,5
The underlying pathogenesis of HE is unknown. Several factors have been postulated, such as mercaptans, short-chain fatty acids, gamma-aminobutyric acid, endorphins, glutamate, endogenous benzodiazepine agonists, tryptophan, zinc deficiency and manganese deposits in the basal ganglia,6 but the most widely known and described key factor for many years now has been increased plasma ammonia.5,7,8 Ammonia crosses the blood–brain barrier and enters the central nervous system, causing astrocyte swelling that directly affects excitatory and inhibitory neurotransmitters. However, no direct correlation has been found between plasma ammonia levels and the degree of HE in patients with cirrhosis, although a correlation has been found with brain ammonia concentrations.9 In fact, high ammonia levels alone have no additional diagnostic or prognostic value in patients with cirrhosis and HE. Nevertheless, this diagnosis must be questioned in the case of normal ammonia levels in a patient with clinical HE.2
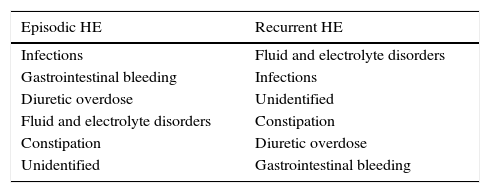
Treatment of hepatic encephalopathyThe first step in the management of HE is to identify possible precipitating factors (Table 1). Treating and controlling these factors is of utmost importance, resulting in the resolution of HE in almost 90% of patients.
Possible precipitating factors for clinical hepatic encephalopathy according to international guidelines2.
| Episodic HE | Recurrent HE |
|---|---|
| Infections | Fluid and electrolyte disorders |
| Gastrointestinal bleeding | Infections |
| Diuretic overdose | Unidentified |
| Fluid and electrolyte disorders | Constipation |
| Constipation | Diuretic overdose |
| Unidentified | Gastrointestinal bleeding |
In order of decreasing frequency.
HE should be treated after detecting and, if possible, correcting the precipitating factor. While many treatments have been proposed, few randomised, controlled trials have been conducted, so many recommendations are currently based on clinical practice.2
The most commonly used and widely reported treatments are non-absorbable disaccharides and non-absorbable or minimally absorbable oral antibiotics, such as rifaximin, although other therapies have been described, including branched-chain amino acids, probiotics and l-ornithine-l-aspartate.
Non-absorbable disaccharides are chosen for 2 reasons. On the one hand, they have a cathartic effect, and on the other, this type of laxative causes acidification of the gut lumen, thus generating a hostile environment for urease-producing bacteria (implicated in the production of intestinal ammonia), reducing ammonia synthesis and, consequently, absorption. Doses of between 30 and 60mg/day are generally used. At these doses it is a safe, generally well tolerated and effective drug in both the treatment and prevention of HE, including minimal HE.10 High doses can sometimes produce side effects such as dehydration and hyponatraemia, which can precipitate or worsen symptoms.
Although lactulose and lactitol are widely used in clinical practice, and many current guidelines continue to recommend them,2 a 2004 meta-analysis showed no effectiveness according to evidence-based medicine criteria.11
Oral antibiotics such as metronidazole, neomycin and rifaximin are another strategy to reduce the amount of ammonia produced by enteric bacteria. Nevertheless, prolonged use of both metronidazole and neomycin can have side effects. Cases of ototoxicity and nephrotoxicity have been reported with aminoglycosides (neomycin and paramomycin), despite their minimal absorption.
Rifaximin is a poorly absorbed bacteriostatic rifamycin derivative that was synthesised in Italy in 1982.12 It has acquired greater importance for the treatment of HE in recent years, and is now the antibiotic of choice due to its good tolerance (few adverse effects) and efficacy.
Several studies have compared rifaximin with placebo, other antibiotics and non-absorbable disaccharide laxatives,13 showing that rifaximin is equal or superior to the different drugs compared, with good tolerance. Longer-term studies have also been performed, comparing it with non-absorbable disaccharides or neomycin as maintenance treatment. A recent study described maintenance treatment with rifaximin vs placebo with free use of non-absorbable disaccharides over 2years of follow-up. The authors found a lower rate of recurrence of HE in patients treated with long-term rifaximin, with no major side effects.14 A meta-analysis carried out this year comparing the efficacy and safety of different treatments for HE also showed a greater reduction in plasma ammonia levels with rifaximin.15
Rational basis for the use of rifaximinRifaximin is a broad spectrum antibiotic with activity against both Gram-positive and Gram-negative bacteria, especially anaerobic enteric bacteria.16 It binds to the beta-subunit of DNA-dependent RNA polymerase, inhibiting RNA synthesis.
The poor intestinal absorption of rifaximin produces high concentrations of the drug in the gastrointestinal tract, which in turn modifies the intestinal bacterial flora. With blood levels of less than 1% after oral administration, rifaximin is safe in healthy patients. However, cirrhosis alters the pharmacokinetics of this drug, with a marked increase in systemic absorption compared with controls. There appears to be a direct relationship with the degree of hepatic insufficiency.17,18
Cirrhotic patients have altered gut microbiota that could affect their cognitive capacity.19 The administration of antibiotics in HE is based on altering the bacterial flora and reducing endotoxaemia by decreasing the production and absorption of gut-derived neurotoxins. Reducing ammonia and endotoxaemia levels could have a positive impact on acute and chronic HE episodes.20,21
Long-term administration of rifaximin seems to have a minimal effect on normal intestinal flora; when used at high doses, an initial decrease in Enterococcus, Escherichia coli, Lactobacillus spp., Bacteroides spp., Bifidobacterium spp. and Clostridium perfringens returns to normal concentrations 1 or 2 weeks12 after discontinuing treatment.22 In a study with 211 patients who received rifaximin for more than 6 months, no Clostridium difficile infections were described,23 although in an earlier study, some cases were reported in at-risk patients treated with rifaximin for 6 months.24 Lastly, Candida albicans, implicated in some cases of antibiotic-associated diarrhoea, was isolated in faecal samples in 20% of patients on treatment with 1200mg/day of rifixamin.12
Resistance to rifaximin is very rare.25 Bacterial resistance was observed to disappear rapidly after stopping a short 5-day course of rifaximin, but no long-term studies have been conducted.18 The selection of resistant mutants of both Gram-negative and Gram-positive bacteria in the gastrointestinal tract is believed to be infrequent in anaerobic conditions, but cannot be completely ruled out.12
Tolerance and side effectsRifaximin is associated with very few side effects. Headache, flatulence, abdominal pain, constipation, nausea and vomiting have been described. These side effects are more minor than those reported with non-absorbable disaccharides, and similar to those presented when compared with placebo.13
Studies conducted with rifaximin at doses of 550mg every 12hours for 6 months24 and more than 2.5years26 showed that the antibiotic was generally well tolerated in all study patients.
Since rifaximin can cause a marked reduction in the faecal Escherichia coli population, long-term treatment could negatively affect coagulation tests, as these bacteria play a role in the production of vitamin K.18
As in the case of rifabutin, rifampicin and rifapentin, hypersensitivity to rifaximin is also possible; cases of urticaria and 1 case of angioneurotic oedema have been reported.18
Finally, as no drug interactions have been reported, rifaximin is considered a very safe drug. Although it induces the enzyme CYP3A4 in vitro, this does not appear to occur in vivo due to its poor oral bioavailability; nevertheless a case of induction of this enzyme in a patient treated with rifaximin for intestinal bacterial overgrowth was reported.27 This interaction interferes with the activity of warfarin. Since patients with cirrhosis have greater absorption of rifaximin with respect to controls, this should be taken into account for possible interactions.18
When should rifaximin be administered?Rifaximin in the treatment of hepatic encephalopathy episodesIn an episode of HE, the primary treatment is to correct/treat the precipitating factor, so it is very important to investigate the possible trigger.
Moreover, the HE can be treated with non-absorbable disaccharides and/or rifaximin, since the severity can range from mild (grade 1) to severe (grade 4 or coma) according to the West Haven classification.2
In 1 prospective, controlled, double blind study on the treatment of acute HE, 120 patients with HE were randomised to receive lactulose combined with 1200mg of rifaximin vs lactulose and placebo.28 Many of these patients were diagnosed with grade 2–4 HE according to West Haven criteria. The patients included had a mean Child-Turcotte-Pugh score of 9.7 and a model for end-stage liver disease (MELD) score of 24.6 points, and were mostly HE grades 3 (33.3%) and 4 (48.3%). All patients received lactulose and rifaximin or placebo through a nasogastric tube and were monitored. The study confirmed lower mortality and a shorter hospital stay in the rifaximin group with respect to patients who received lactulose together with placebo. In the latter group, deaths were due to sepsis, so adding rifaximin in the treatment of a severe episode of HE is more effective than the administration of non-absorbable disaccharides alone.
Other randomised studies have assessed the role of rifaximin, showing that it is more effective than non-absorbable disaccharides and has the same or greater efficacy than other antibiotics used in patients with mild to severe encephalopathy.17,29,32
Many studies published in recent years favour the use of rifaximin in acute episodes of HE28,30,32–42 as well as in the prevention of recurrence14,24,26,31,43–48 and in minimal HE25,39,50–55; most were associated with the free use of non-absorbable disaccharides. Finally, several review articles or meta-analyses have been published suggesting that rifaximin is at least as effective as non-absorbable disaccharides, and is better tolerated.56,57 Statistically, however, it has not been found to be superior; therefore, its use as monotherapy for the treatment and prevention of episodes of recurrent HE is still not recommended in international guidelines, although it is considered an effective add-on treatment to non-absorbable disaccharides2,56 (Tables 2–4).
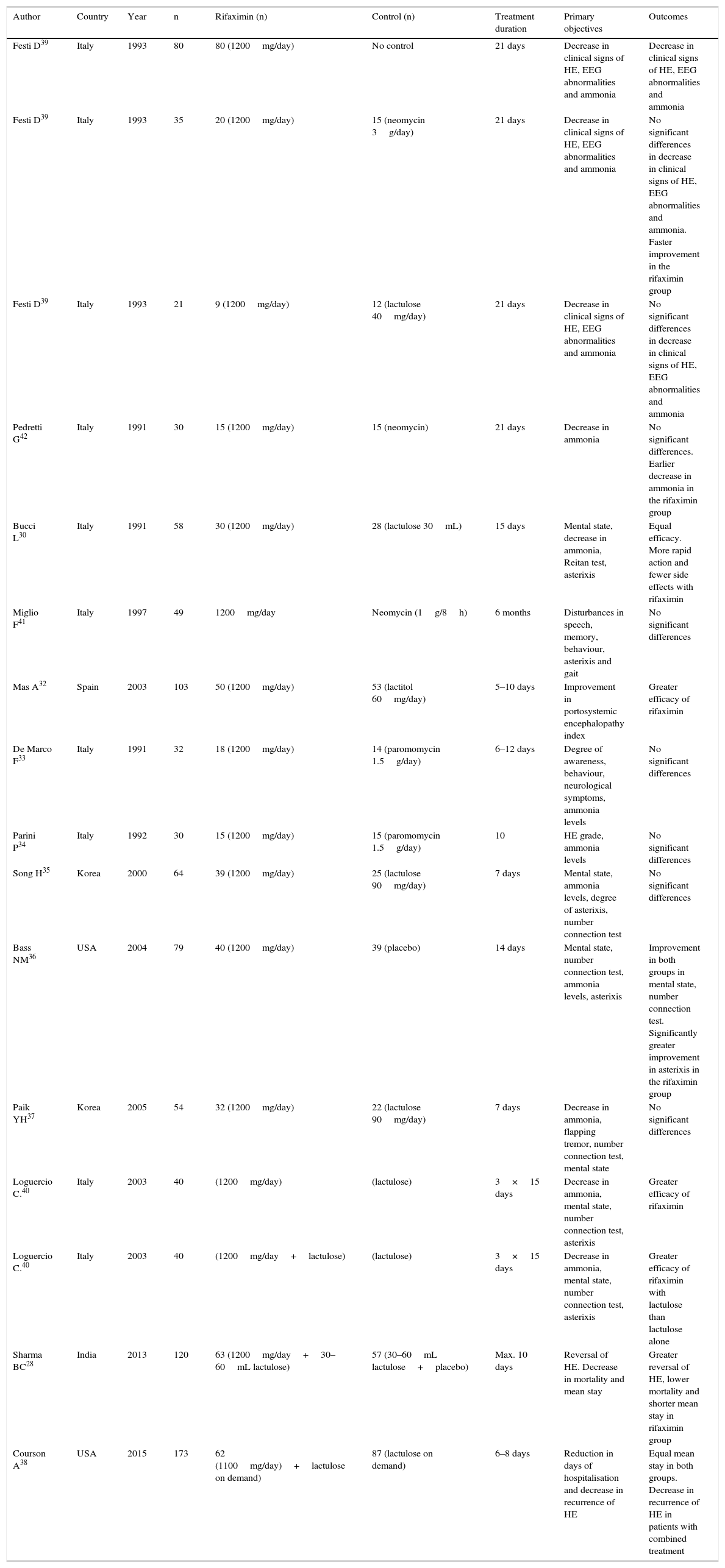
Rifaximin in the treatment of hepatic encephalopathy (HE) episodes.
| Author | Country | Year | n | Rifaximin (n) | Control (n) | Treatment duration | Primary objectives | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Festi D39 | Italy | 1993 | 80 | 80 (1200mg/day) | No control | 21 days | Decrease in clinical signs of HE, EEG abnormalities and ammonia | Decrease in clinical signs of HE, EEG abnormalities and ammonia |
| Festi D39 | Italy | 1993 | 35 | 20 (1200mg/day) | 15 (neomycin 3g/day) | 21 days | Decrease in clinical signs of HE, EEG abnormalities and ammonia | No significant differences in decrease in clinical signs of HE, EEG abnormalities and ammonia. Faster improvement in the rifaximin group |
| Festi D39 | Italy | 1993 | 21 | 9 (1200mg/day) | 12 (lactulose 40mg/day) | 21 days | Decrease in clinical signs of HE, EEG abnormalities and ammonia | No significant differences in decrease in clinical signs of HE, EEG abnormalities and ammonia |
| Pedretti G42 | Italy | 1991 | 30 | 15 (1200mg/day) | 15 (neomycin) | 21 days | Decrease in ammonia | No significant differences. Earlier decrease in ammonia in the rifaximin group |
| Bucci L30 | Italy | 1991 | 58 | 30 (1200mg/day) | 28 (lactulose 30mL) | 15 days | Mental state, decrease in ammonia, Reitan test, asterixis | Equal efficacy. More rapid action and fewer side effects with rifaximin |
| Miglio F41 | Italy | 1997 | 49 | 1200mg/day | Neomycin (1g/8h) | 6 months | Disturbances in speech, memory, behaviour, asterixis and gait | No significant differences |
| Mas A32 | Spain | 2003 | 103 | 50 (1200mg/day) | 53 (lactitol 60mg/day) | 5–10 days | Improvement in portosystemic encephalopathy index | Greater efficacy of rifaximin |
| De Marco F33 | Italy | 1991 | 32 | 18 (1200mg/day) | 14 (paromomycin 1.5g/day) | 6–12 days | Degree of awareness, behaviour, neurological symptoms, ammonia levels | No significant differences |
| Parini P34 | Italy | 1992 | 30 | 15 (1200mg/day) | 15 (paromomycin 1.5g/day) | 10 | HE grade, ammonia levels | No significant differences |
| Song H35 | Korea | 2000 | 64 | 39 (1200mg/day) | 25 (lactulose 90mg/day) | 7 days | Mental state, ammonia levels, degree of asterixis, number connection test | No significant differences |
| Bass NM36 | USA | 2004 | 79 | 40 (1200mg/day) | 39 (placebo) | 14 days | Mental state, number connection test, ammonia levels, asterixis | Improvement in both groups in mental state, number connection test. Significantly greater improvement in asterixis in the rifaximin group |
| Paik YH37 | Korea | 2005 | 54 | 32 (1200mg/day) | 22 (lactulose 90mg/day) | 7 days | Decrease in ammonia, flapping tremor, number connection test, mental state | No significant differences |
| Loguercio C.40 | Italy | 2003 | 40 | (1200mg/day) | (lactulose) | 3×15 days | Decrease in ammonia, mental state, number connection test, asterixis | Greater efficacy of rifaximin |
| Loguercio C.40 | Italy | 2003 | 40 | (1200mg/day+lactulose) | (lactulose) | 3×15 days | Decrease in ammonia, mental state, number connection test, asterixis | Greater efficacy of rifaximin with lactulose than lactulose alone |
| Sharma BC28 | India | 2013 | 120 | 63 (1200mg/day+30–60mL lactulose) | 57 (30–60mL lactulose+placebo) | Max. 10 days | Reversal of HE. Decrease in mortality and mean stay | Greater reversal of HE, lower mortality and shorter mean stay in rifaximin group |
| Courson A38 | USA | 2015 | 173 | 62 (1100mg/day)+lactulose on demand) | 87 (lactulose on demand) | 6–8 days | Reduction in days of hospitalisation and decrease in recurrence of HE | Equal mean stay in both groups. Decrease in recurrence of HE in patients with combined treatment |
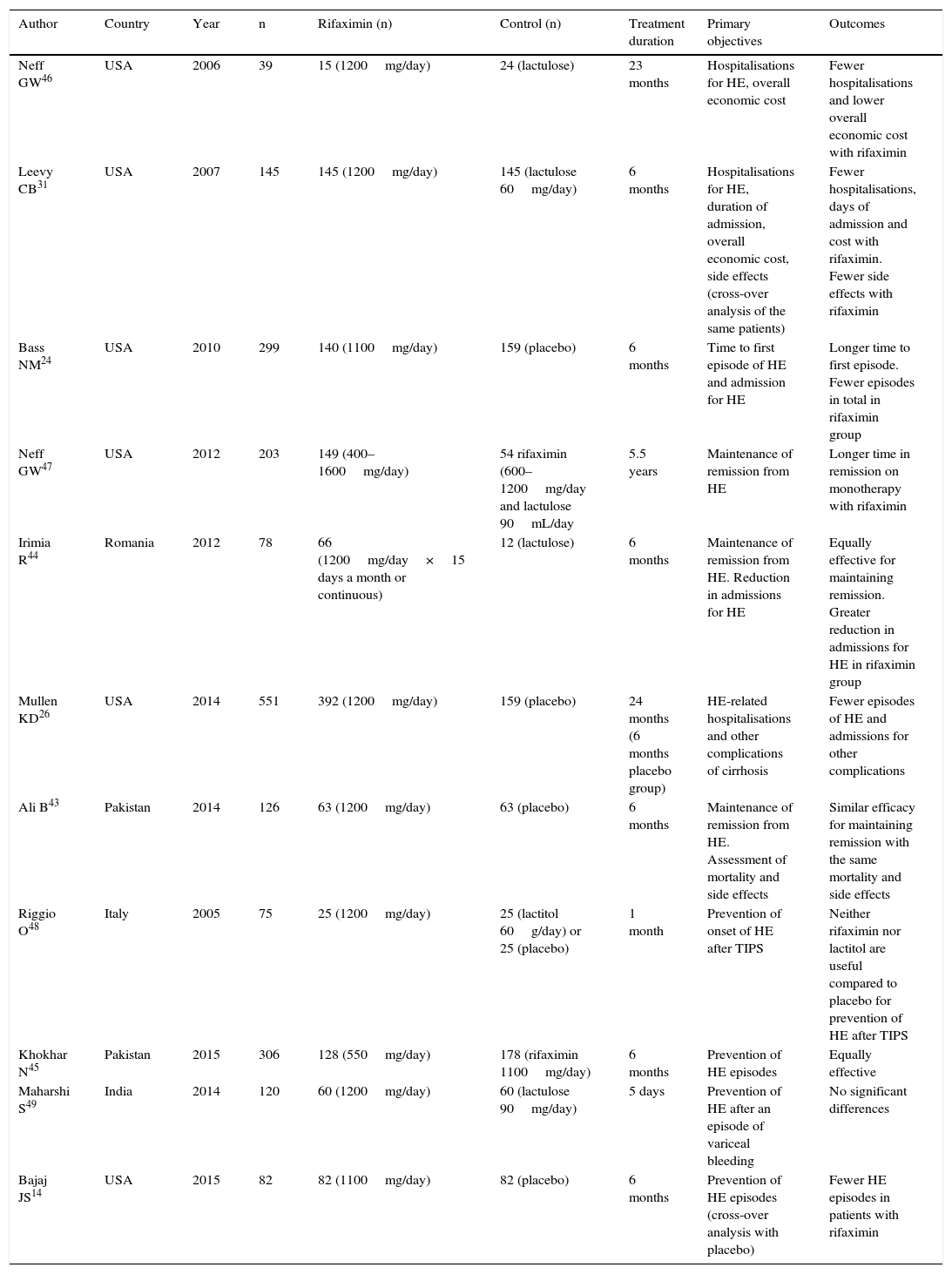
Rifaximin in the prevention of hepatic encephalopathy (HE) recurrence.
| Author | Country | Year | n | Rifaximin (n) | Control (n) | Treatment duration | Primary objectives | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Neff GW46 | USA | 2006 | 39 | 15 (1200mg/day) | 24 (lactulose) | 23 months | Hospitalisations for HE, overall economic cost | Fewer hospitalisations and lower overall economic cost with rifaximin |
| Leevy CB31 | USA | 2007 | 145 | 145 (1200mg/day) | 145 (lactulose 60mg/day) | 6 months | Hospitalisations for HE, duration of admission, overall economic cost, side effects (cross-over analysis of the same patients) | Fewer hospitalisations, days of admission and cost with rifaximin. Fewer side effects with rifaximin |
| Bass NM24 | USA | 2010 | 299 | 140 (1100mg/day) | 159 (placebo) | 6 months | Time to first episode of HE and admission for HE | Longer time to first episode. Fewer episodes in total in rifaximin group |
| Neff GW47 | USA | 2012 | 203 | 149 (400–1600mg/day) | 54 rifaximin (600–1200mg/day and lactulose 90mL/day | 5.5 years | Maintenance of remission from HE | Longer time in remission on monotherapy with rifaximin |
| Irimia R44 | Romania | 2012 | 78 | 66 (1200mg/day×15 days a month or continuous) | 12 (lactulose) | 6 months | Maintenance of remission from HE. Reduction in admissions for HE | Equally effective for maintaining remission. Greater reduction in admissions for HE in rifaximin group |
| Mullen KD26 | USA | 2014 | 551 | 392 (1200mg/day) | 159 (placebo) | 24 months (6 months placebo group) | HE-related hospitalisations and other complications of cirrhosis | Fewer episodes of HE and admissions for other complications |
| Ali B43 | Pakistan | 2014 | 126 | 63 (1200mg/day) | 63 (placebo) | 6 months | Maintenance of remission from HE. Assessment of mortality and side effects | Similar efficacy for maintaining remission with the same mortality and side effects |
| Riggio O48 | Italy | 2005 | 75 | 25 (1200mg/day) | 25 (lactitol 60g/day) or 25 (placebo) | 1 month | Prevention of onset of HE after TIPS | Neither rifaximin nor lactitol are useful compared to placebo for prevention of HE after TIPS |
| Khokhar N45 | Pakistan | 2015 | 306 | 128 (550mg/day) | 178 (rifaximin 1100mg/day) | 6 months | Prevention of HE episodes | Equally effective |
| Maharshi S49 | India | 2014 | 120 | 60 (1200mg/day) | 60 (lactulose 90mg/day) | 5 days | Prevention of HE after an episode of variceal bleeding | No significant differences |
| Bajaj JS14 | USA | 2015 | 82 | 82 (1100mg/day) | 82 (placebo) | 6 months | Prevention of HE episodes (cross-over analysis with placebo) | Fewer HE episodes in patients with rifaximin |
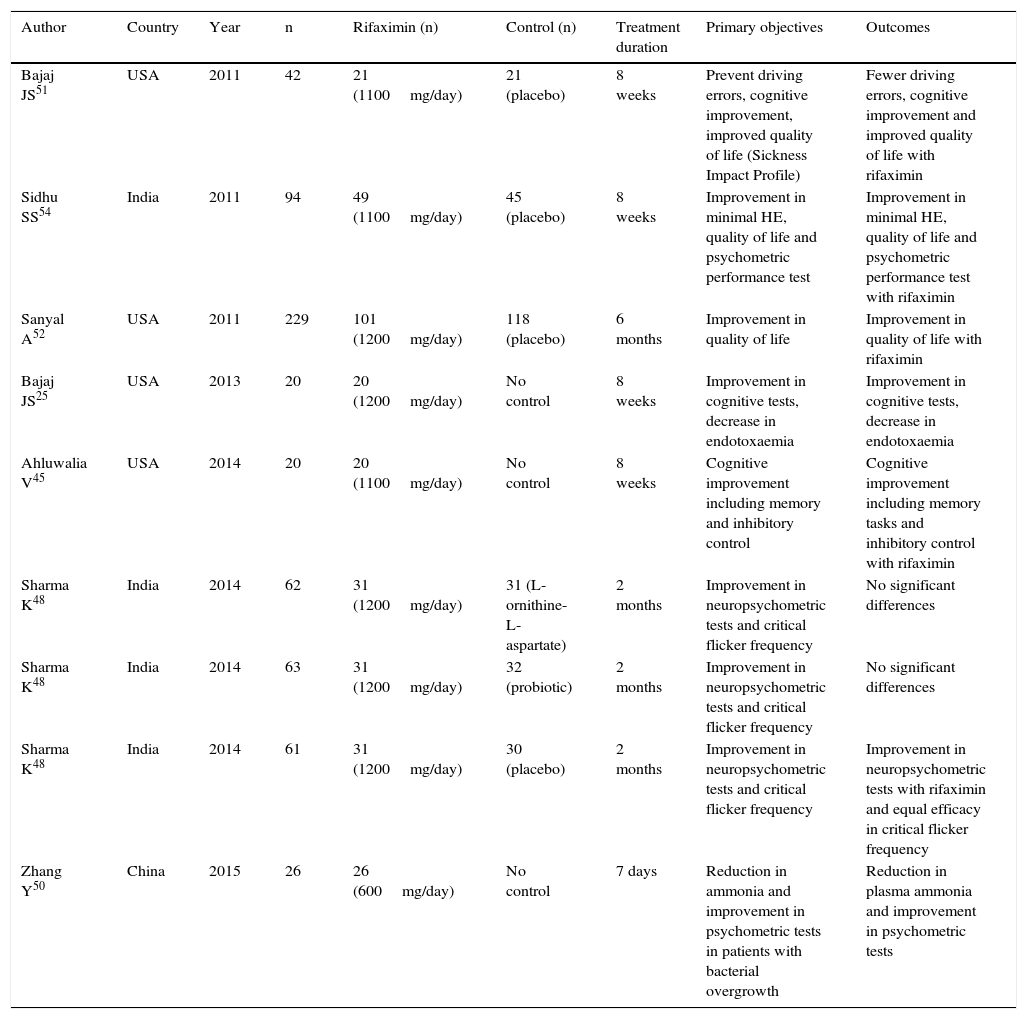
Rifaximin in minimal hepatic encephalopathy (HE).
| Author | Country | Year | n | Rifaximin (n) | Control (n) | Treatment duration | Primary objectives | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Bajaj JS51 | USA | 2011 | 42 | 21 (1100mg/day) | 21 (placebo) | 8 weeks | Prevent driving errors, cognitive improvement, improved quality of life (Sickness Impact Profile) | Fewer driving errors, cognitive improvement and improved quality of life with rifaximin |
| Sidhu SS54 | India | 2011 | 94 | 49 (1100mg/day) | 45 (placebo) | 8 weeks | Improvement in minimal HE, quality of life and psychometric performance test | Improvement in minimal HE, quality of life and psychometric performance test with rifaximin |
| Sanyal A52 | USA | 2011 | 229 | 101 (1200mg/day) | 118 (placebo) | 6 months | Improvement in quality of life | Improvement in quality of life with rifaximin |
| Bajaj JS25 | USA | 2013 | 20 | 20 (1200mg/day) | No control | 8 weeks | Improvement in cognitive tests, decrease in endotoxaemia | Improvement in cognitive tests, decrease in endotoxaemia |
| Ahluwalia V45 | USA | 2014 | 20 | 20 (1100mg/day) | No control | 8 weeks | Cognitive improvement including memory and inhibitory control | Cognitive improvement including memory tasks and inhibitory control with rifaximin |
| Sharma K48 | India | 2014 | 62 | 31 (1200mg/day) | 31 (L-ornithine-L-aspartate) | 2 months | Improvement in neuropsychometric tests and critical flicker frequency | No significant differences |
| Sharma K48 | India | 2014 | 63 | 31 (1200mg/day) | 32 (probiotic) | 2 months | Improvement in neuropsychometric tests and critical flicker frequency | No significant differences |
| Sharma K48 | India | 2014 | 61 | 31 (1200mg/day) | 30 (placebo) | 2 months | Improvement in neuropsychometric tests and critical flicker frequency | Improvement in neuropsychometric tests with rifaximin and equal efficacy in critical flicker frequency |
| Zhang Y50 | China | 2015 | 26 | 26 (600mg/day) | No control | 7 days | Reduction in ammonia and improvement in psychometric tests in patients with bacterial overgrowth | Reduction in plasma ammonia and improvement in psychometric tests |
The current recommendation for the treatment of an episode of HE is the lactulose or lactitol of choice, adding rifaximin in patients who do not respond to non-absorbable disaccharides after 24–48hours,2 as well as correcting possible precipitating factors (Table 5).
Current treatment recommendations.
| Acute episode | • Correct precipitating factors • Treatment with non-absorbable disaccharides • Initiate rifaximin if no improvement 48–72h after starting lactulose/lactitol treatment • If the patient was previously on this treatment, start rifaximin on admission |
| Recurrent HE | • Administer non-absorbable disaccharide laxatives as prophylaxis after a first episode of HE • If patient has a new episode whilst on lactulose/lactitol treatment, add rifaximin (starting dose 1200mg/day) |
| Minimal HE | • No treatment currently recommended |
| Placement TIPS | • Prophylaxis not routinely recommended |
TIPS: transjugular intrahepatic portosystemic shunt.
Recurrent HE is defined as more than 2 episodes of HE in 1 year.4
One of the pivotal studies on this drug was conducted in patients with recurrent HE.24 This was a 6-month randomised, double-blind, placebo-controlled study in patients with cirrhosis, with free use of non-absorbable disaccharides. It showed that rifaximin reduced the risk of HE recurrences (22.1% vs 45.9% in the placebo group) and hospitalisation related with this decompensation: 13.6% in the group treated with rifaximin compared to 22.6% in the placebo group. This clinical improvement also led to better quality of life, with an incidence of side effects comparable to placebo. With regard to the use of non-absorbable disaccharides, almost all patients, both in the rifaximin and the placebo group, received lactulose (91.4% compared to 91.2%, respectively). However, patients in clinical practice generally require more long-term treatments (longer than 6 months). As described above, no studies to date have assessed the continuous administration of rifaximin. A recent study assessed administration of rifaximin over an average of 24 months. This study demonstrated both the safety (few adverse effects) and efficacy of this drug, based on the number of HE episodes and the lower rate of readmission for both HE and other causes. Although other reasons for hospitalisation (apart from HE) were not mentioned, the authors postulated that administering rifaximin and reducing endotoxaemia might have a role in the decrease of cytokines such as interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α) and nitric acid,58 which could result in better clinical progress.
To assess the reproducibility and, therefore, the reliability of the results of previous studies, the efficacy of administration of rifaximin was assessed in patients who, in the study on rifaximin vs placebo for 6 months, had been treated with placebo. The results showed that when these patients were switched from placebo to rifaximin, more than 65% were protected against recurrent HE episodes.14
A unique situation is HE in patients with TIPS. Standard treatment is generally used to prevent HE episodes after placement of the shunt. Nevertheless, 1 study showed that neither lactulose nor rifaximin administration was more effective in preventing post-TIPS episodes of HE compared to placebo.48 Since patients who are candidates for TIPS are now more carefully selected, the incidence of severe HE after shunt insertion has been reduced. If this does occur, however, reducing the diameter of the shunt, which can reverse HE, should be considered.59
Current recommendations for recurrent HE, therefore, involve administering lactulose after the first episode as prophylaxis against a new episode. If the patient presents symptoms of HE whilst on this treatment, rifaximin should be added (starting dose of 1200mg/day, which can be reduced to 800mg/day). Routine prophylaxis against HE with lactulose or rifaximin is not recommended after placement of a TIPS.2
Rifaximin in minimal hepatic encephalopathyMinimal HE is a form of HE that leads to a mild neurological or cognitive deficit that can only be detected by advanced neuro-psychological tests. Studies have shown rifaximin and lactulose to be effective in this type of encephalopathy and in improving patient quality of life.52,54 One randomised controlled trial showed that administration of rifaximin in patients with HE significantly improved driving simulator performance when compared with placebo.51 Specifically, 8 weeks after starting the study, patients treated with rifaximin showed a significantly greater improvement in avoiding driving errors (76% vs 31%, p=0.013), speeding (81% vs 33%, p=0.005) and illegal turns (62% vs 19%, p=0.01) compared to those receiving placebo. A significant improvement was also observed in cognitive performance (91% vs 61%, p=0.01) and in quality of life (p=0.04) in patients treated with rifaximin compared to controls.
Given the variety of methods used to define minimal HE, the objectives and short duration of the studies, no routine treatment is currently recommended in minimal HE.
Cost-effectiveness of rifaximinPharmaco-economic analyses are always limited, complex and difficult to evaluate; furthermore, the result obtained may not be valid in other geographical regions due to differences in the healthcare system, medical practice60 and drug cost (intra-country variations).
A pragmatic approach to the issue is to include the cost of the drugs as a saving if negative outcomes are avoided, such as a reduction in hospitalisation rates.13 In a retrospective study with 39 patients, the cost per person and year was lower if patients received rifaximin treatment than if they received lactulose treatment.46 One study evaluated competing strategies to assess the most cost-effective option for treatment of HE. The arms were: (1) no HE treatment; (2) lactulose monotherapy; (3) lactitol monotherapy; (4) neomycin monotherapy; (5) rifaximin monotherapy, and (6) lactulose and rifaximin salvage if intolerance or no response to treatment. The study showed that monotherapy with rifaximin was not cost-effective based on the price. In contrast, monotherapy with lactulose and the rifaximin salvage treatment (joint administration of both) in patients who did not initially respond to lactulose was shown to be cost-effective.61
Although rifaximin is more expensive than lactulose or lactitol, there is evidence to support its use in preventing new admissions for encephalopathy or other complications of cirrhosis. The use of rifaximin could reduce healthcare costs.61 A recent cost-effectiveness analysis based on the United Kingdom healthcare service, using a Markov model, estimated that rifaximin was cost-effective compared with standard treatment at 5 and 10 years, and even in the case of lifelong treatment.60
In cases of minimal HE, however, where few patients are hospitalised and therapy is often prolonged, it does not appear to be cost-effective.
Treatment adherenceTwo studies have reported good therapeutic adherence with rifaximin: between 84% and 92%.24,51 This is considered very high in relation to the level of adherence described in some studies in lactulose (54% adherence).62 In a retrospective study in which patients received rifaximin and lactulose for 6 months, “compliance” was defined as adherence with at least 75% of medication. The results showed that 92% of patients in the rifaximin group and 31% of patients in the lactulose group were compliant.31 Factors that could be involved in poorer treatment adherence could be fatigue, confusion, lack of social support and a complicated dosage regimen.63
ConclusionsRifaximin has been shown to be effective in the acute treatment and prevention of HE. It is better tolerated than non-absorbable disaccharides. Rifaximin is more effective in reducing blood ammonia levels, which leads to fewer and shorter hospitalisations and lower hospital-related costs. Baseline treatment with non-absorbable disaccharides and salvage treatment with rifaximin in patients who do not initially respond to lactulose/lactitol has been confirmed as cost-effective, although some aspects remain to be clarified. There is no clear evidence to support the use of rifaximin in the treatment of minimal HE.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Sanchez-Delgado J, Miquel M. Papel de la rifaximina en el tratamiento de la encefalopatía hepática. Gastroenterol Hepatol. 2016;39:282–292.