This study examines the indications according to which antiepileptic drugs are prescribed and used in a population of patients enrolled in the Colombian national health system (SGSSS).
MethodsRetrospective cross-sectional study. From the pool of individuals in 34 Colombian cities who used antiepileptic drugs between 18 July 2013 and 31 August 2014 during a period of no less than 12 months, we obtained a random sample stratified by city. Socio-demographic, pharmacological and comorbidity variables were analysed. Continuous and categorical variables were compared, and logistic regression models were used.
ResultsOur patient total was 373 patients, with 197 women (52.1%) and a mean age of 41.9±21.7years; 65.4% of the patients were treated with monotherapy. The most frequently used drugs were valproic acid (53.1%) and carbamazepine (33.2%). Epilepsy was the most frequent indication (n=178; 47.7%); however, 52.3% of the patients were prescribed antiepileptics for different indications, especially neuropathic pain (26.8%), affective disorders (14.2%) and migraine prophylaxis (12.3%). A total of 81 patients with epilepsy (46.6%) displayed good seizure control while another 25 (14.4%) had drug-resistant epilepsy. In the multivariate analysis, medication adherence was associated with a lower risk of treatment failure in patients with epilepsy (OR: 0.27; 95% CI: 0.11-0.67).
ConclusionsIn Colombia, antiepileptic drugs are being used for indications other than those originally intended. Monotherapy is the most commonly used treatment approach, together with the use of classic antiepileptic drugs.
Determinar las indicaciones por las cuales se prescriben y utilizan antiepilépticos en una población de pacientes afiliados al Sistema General de Seguridad Social en Salud (SGSSS) de Colombia.
MétodosEstudio retrospectivo de corte transversal. A partir de todos los individuos que utilizaron antiepilépticos entre el 18 de julio de 2013 y el 31 de agosto de 2014 en 34 ciudades colombianas durante un periodo no inferior a 12 meses, se realizó un muestreo aleatorizado estratificado por ciudades. Se analizaron variables sociodemográficas, farmacológicas y comorbilidades. Se compararon variables continuas y categóricas, y se realizaron modelos de regresión logística.
ResultadosDe un total de 373 sujetos, se hallaron 197 mujeres (52,1%); el promedio de edad fue de 41,9±21,7años; predominó la monoterapia en el 65,4% de los pacientes. Los medicamentos más utilizados fueron ácido valproico (53,1%) y carbamazepina (33,2%). La epilepsia fue la indicación más frecuente (n=178; 47,7%); sin embargo, en el 52,3% de pacientes se utilizaron para indicaciones diferentes, especialmente dolor neuropático (26,8%), trastornos afectivos (14,2%) y profilaxis de migraña (12,3%). Un total de 81 pacientes con epilepsia (46,6%) estaban en control sintomático, mientras otros 25 casos (14,4%) presentaban epilepsia resistente a fármacos. En el análisis multivariado la adherencia al tratamiento se asoció con menor riesgo de fracaso terapéutico en pacientes con epilepsia (OR: 0,27; IC95%: 0,11-0,67).
ConclusionesLos fármacos antiepilépticos en Colombia se están utilizando en indicaciones diferentes para las que fueron inicialmente diseñados. La monoterapia es la estrategia tera-péutica más empleada, al igual que el uso de medicamentos clásicos dentro del grupo.
Antiepileptic drugs are the cornerstone of epilepsy treatment; around 70% of patients with this disorder become seizure-free with medication and can lead a normal life with no adverse drug events.1
Antiepileptic drugs can be classified either chronologically, as conventional (first-generation) or new (second- and third-generation) drugs, or by their action mechanism. However, the latter classification may be challenging since many of these drugs act via more than one action mechanism, including blocking sodium and calcium channels, enhancing GABA activity, reducing excitation mediated by NMDA (glutamate) receptors, and inhibiting neurotransmitter release.2–4 Due to their multiple mechanisms of action on the nervous system, these drugs are frequently used for conditions other than epilepsy.3,5
The Food and Drug Administration (FDA) recently approved use of these drugs for treating several forms of chronic and neuropathic pain, such as trigeminal and postherpetic neuralgia, fibromyalgia, migraine, and multiple psychiatric disorders including bipolar affective disorder. In addition, despite not having FDA approval for these uses, antiepileptics have been proved effective for treating hyperkinetic movement disorders, schizophrenia, drug addiction, autism, tinnitus, and others.3,5
Approximately 40% of patients taking antiepileptics may experience adverse drug effects; this is a major cause of treatment failure not only because patients abandon treatment in early stages but also because adverse effects affect adherence, resulting in failure to reach the desired effect of full doses.6
In this context, and considering that there are no studies on antiepileptic use in Colombia, we aimed to determine how these drugs are actually used. Here, we analyse such variables as reason for prescription, dose, adherence, changes in pharmacological treatment, types of reported adverse effects, and results of pharmacological treatment (effectiveness). Our purpose was to provide a deeper knowledge of antiepileptic use in Colombia, which may be of help in developing programmes that achieve better treatments and appropriate use of antiepileptic drugs.
Materials and methodsWe conducted a retrospective cross-sectional study of the reasons for prescribing antiepileptic drugs in Colombia. We included data from all patients registered with a health insurance provider (HIP) and affiliated with the Colombian health and social security system (SGSSS). Patients had been receiving antiepileptics for at least 12 months and were seen by a doctor between 18 July 2013 and 31 August 2014 in one of 34 Colombian cities, including Barranquilla, Bogotá, Bucaramanga, Cali, Cartagena, Ibagué, Manizales, Medellín, Pereira, and Santa Marta.
Randomised sampling stratified by city was conducted based on a total of 12736 patients receiving treatment to establish the sample size with a maximum margin of error of 5% and a 95% confidence interval.
A final-year medical student reviewed all medical histories that had been recorded by the HIP. Our team designed a database that was validated and reviewed by a pharmacologist and which enabled us to enter the following data during the observation period:
- (1)
Sociodemographic variables: age, sex, city of residence.
- (2)
Clinical variables: clinical diagnosis motivating prescription of the antiepileptic drug, seizure-free and symptom-free interval, adverse drug effects, changes in medication or dose, and adherence to treatment. We defined treatment failure as occurrence of seizures in one year. Drug-resistant epilepsy was defined as lack of seizure control despite using 2 tolerated and appropriately chosen antiepileptic drugs provoking no adverse effects (whether as monotherapies or in combination) during one year.7
- (3)
Laboratory variables: haemoglobin, white blood cell count, and serum levels of hepatic enzymes, bilirubin, sodium, and antiepileptic agents.
- (4)
Antiepileptic drugs administered and dose. We included those drugs available in Colombia and recorded whether they were prescribed as monotherapies or in combination therapy.
- (5)
The following comorbidities and associated treatments were also entered: (a) diabetes mellitus; (b) Parkinson's disease; (c) HIV-AIDS; (d) depression; (e) anxiety or sleep disorders; (f) dyslipidaemia; (g) hypothyroidism; (h) ischaemic cardiomyopathy; (i) arterial hypertension; (j) bipolar affective disorder; (k) psychoses/schizophrenia; (l) pain; (m) acid peptic disease; and (n) allergies. Potential risks for drug interactions were also considered.
Our study protocol was classified by the bioethics committee of the Universidad Tecnológica de Pereira as ‘research with no risk’ according to Resolution 8430/1993 issued by the Colombian Ministry of Health in compliance with the Declaration of Helsinki. Confidentiality of patient data was preserved.
The statistical analysis was completed using SPSS statistical software version 22.0 (IBM, US) for Windows. We used the t-test or ANOVA method for quantitative variables and the chi-square test for categorical variables. A logistic regression model was applied using treatment failure in epilepsy patients as the dependent variable; the covariates were those variables showing a significant association with the dependent variable in bivariate analyses. The level of statistical significance was set at P<.05.
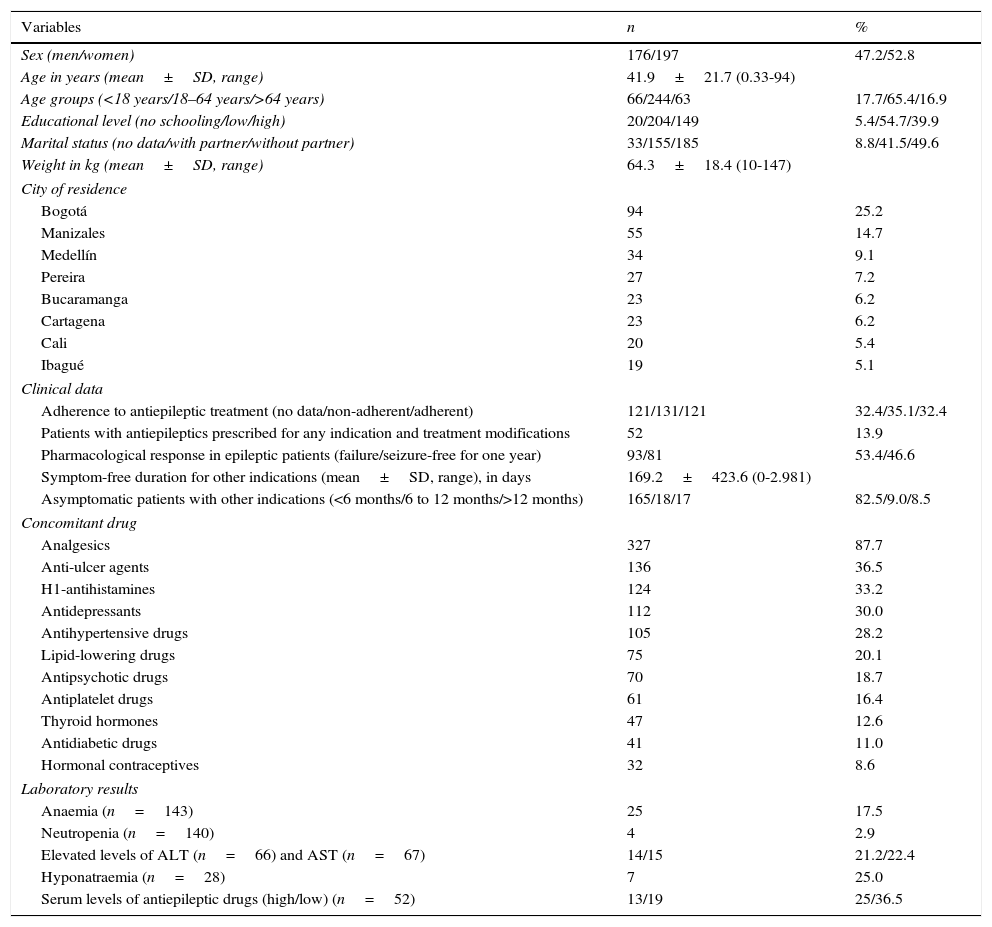
ResultsWe obtained a total of 373 patients of whom 197 (52.1%) were women (4 were pregnant). Men accounted for 47.9% of the total (176). Mean age was 41.9±21.7 years (range, 4 months to 94 years). Table 1 summarises sociodemographic and clinical characteristics, concomitant drug data, and laboratory results in the 373 patients receiving antiepileptic drugs during the study period.
Sociodemographic and clinical characteristics, concomitant drug data, and laboratory findings from 373 patients treated with antiepileptic drugs in Colombia in 2014.
| Variables | n | % |
|---|---|---|
| Sex (men/women) | 176/197 | 47.2/52.8 |
| Age in years (mean±SD, range) | 41.9±21.7 (0.33-94) | |
| Age groups (<18 years/18–64 years/>64 years) | 66/244/63 | 17.7/65.4/16.9 |
| Educational level (no schooling/low/high) | 20/204/149 | 5.4/54.7/39.9 |
| Marital status (no data/with partner/without partner) | 33/155/185 | 8.8/41.5/49.6 |
| Weight in kg (mean±SD, range) | 64.3±18.4 (10-147) | |
| City of residence | ||
| Bogotá | 94 | 25.2 |
| Manizales | 55 | 14.7 |
| Medellín | 34 | 9.1 |
| Pereira | 27 | 7.2 |
| Bucaramanga | 23 | 6.2 |
| Cartagena | 23 | 6.2 |
| Cali | 20 | 5.4 |
| Ibagué | 19 | 5.1 |
| Clinical data | ||
| Adherence to antiepileptic treatment (no data/non-adherent/adherent) | 121/131/121 | 32.4/35.1/32.4 |
| Patients with antiepileptics prescribed for any indication and treatment modifications | 52 | 13.9 |
| Pharmacological response in epileptic patients (failure/seizure-free for one year) | 93/81 | 53.4/46.6 |
| Symptom-free duration for other indications (mean±SD, range), in days | 169.2±423.6 (0-2.981) | |
| Asymptomatic patients with other indications (<6 months/6 to 12 months/>12 months) | 165/18/17 | 82.5/9.0/8.5 |
| Concomitant drug | ||
| Analgesics | 327 | 87.7 |
| Anti-ulcer agents | 136 | 36.5 |
| H1-antihistamines | 124 | 33.2 |
| Antidepressants | 112 | 30.0 |
| Antihypertensive drugs | 105 | 28.2 |
| Lipid-lowering drugs | 75 | 20.1 |
| Antipsychotic drugs | 70 | 18.7 |
| Antiplatelet drugs | 61 | 16.4 |
| Thyroid hormones | 47 | 12.6 |
| Antidiabetic drugs | 41 | 11.0 |
| Hormonal contraceptives | 32 | 8.6 |
| Laboratory results | ||
| Anaemia (n=143) | 25 | 17.5 |
| Neutropenia (n=140) | 4 | 2.9 |
| Elevated levels of ALT (n=66) and AST (n=67) | 14/15 | 21.2/22.4 |
| Hyponatraemia (n=28) | 7 | 25.0 |
| Serum levels of antiepileptic drugs (high/low) (n=52) | 13/19 | 25/36.5 |
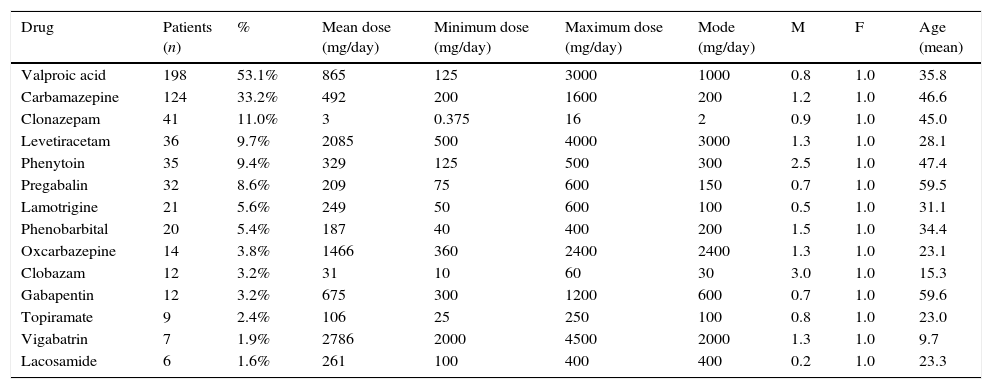
The most frequently prescribed drug was valproic acid, followed by carbamazepine. We should also point out that vigabatrin, lamotrigine, and oxcarbazepine were administered at doses greater than the maximum recommended dose. None of the study participants had been prescribed acetazolamide, primidone, ethosuximide, rufinamide, or felbamate. Patients were predominantly treated in monotherapy (n=244; 65.4%); two drugs were administered to 21.2% of patients (n=79), 3% to 10.5% (n=39), 4% to 2.4% (n=9), and 6% to 0.5% (n=2). Table 2 shows prescription patterns for the antiepileptics used by the study patients.
Prescription pattern for antiepileptics taken by 373 patients registered with a HIP within the Colombian health system in 2014.
| Drug | Patients (n) | % | Mean dose (mg/day) | Minimum dose (mg/day) | Maximum dose (mg/day) | Mode (mg/day) | M | F | Age (mean) |
|---|---|---|---|---|---|---|---|---|---|
| Valproic acid | 198 | 53.1% | 865 | 125 | 3000 | 1000 | 0.8 | 1.0 | 35.8 |
| Carbamazepine | 124 | 33.2% | 492 | 200 | 1600 | 200 | 1.2 | 1.0 | 46.6 |
| Clonazepam | 41 | 11.0% | 3 | 0.375 | 16 | 2 | 0.9 | 1.0 | 45.0 |
| Levetiracetam | 36 | 9.7% | 2085 | 500 | 4000 | 3000 | 1.3 | 1.0 | 28.1 |
| Phenytoin | 35 | 9.4% | 329 | 125 | 500 | 300 | 2.5 | 1.0 | 47.4 |
| Pregabalin | 32 | 8.6% | 209 | 75 | 600 | 150 | 0.7 | 1.0 | 59.5 |
| Lamotrigine | 21 | 5.6% | 249 | 50 | 600 | 100 | 0.5 | 1.0 | 31.1 |
| Phenobarbital | 20 | 5.4% | 187 | 40 | 400 | 200 | 1.5 | 1.0 | 34.4 |
| Oxcarbazepine | 14 | 3.8% | 1466 | 360 | 2400 | 2400 | 1.3 | 1.0 | 23.1 |
| Clobazam | 12 | 3.2% | 31 | 10 | 60 | 30 | 3.0 | 1.0 | 15.3 |
| Gabapentin | 12 | 3.2% | 675 | 300 | 1200 | 600 | 0.7 | 1.0 | 59.6 |
| Topiramate | 9 | 2.4% | 106 | 25 | 250 | 100 | 0.8 | 1.0 | 23.0 |
| Vigabatrin | 7 | 1.9% | 2786 | 2000 | 4500 | 2000 | 1.3 | 1.0 | 9.7 |
| Lacosamide | 6 | 1.6% | 261 | 100 | 400 | 400 | 0.2 | 1.0 | 23.3 |
F: female; M: male.
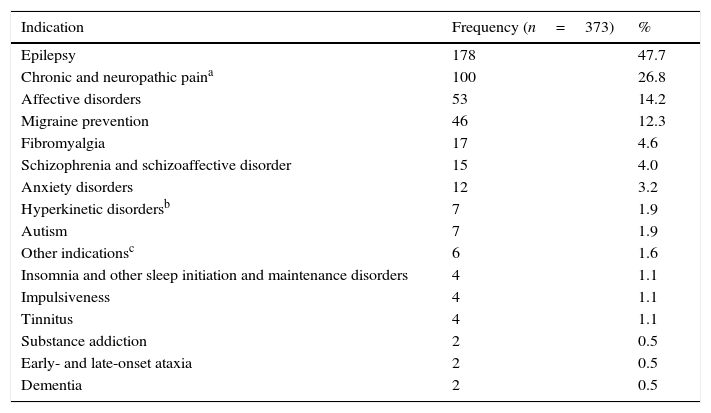
While epilepsy was the most common reason for prescribing antiepileptics, we should highlight that more than half of the patients received these drugs to treat other entities. Table 3 lists the conditions for which antiepileptics were prescribed. Whereas 308 patients (82.5%) received the medication for one condition only, 50 patients (13.4%) were treated for 2 conditions, 10 patients (2.6%) for 3, 4 patients (1.0%) for 4, and one patient for 5 different conditions.
Reasons for prescribing antiepileptics in 373 patients covered by a HIP in Colombia in 2014.
| Indication | Frequency (n=373) | % |
|---|---|---|
| Epilepsy | 178 | 47.7 |
| Chronic and neuropathic paina | 100 | 26.8 |
| Affective disorders | 53 | 14.2 |
| Migraine prevention | 46 | 12.3 |
| Fibromyalgia | 17 | 4.6 |
| Schizophrenia and schizoaffective disorder | 15 | 4.0 |
| Anxiety disorders | 12 | 3.2 |
| Hyperkinetic disordersb | 7 | 1.9 |
| Autism | 7 | 1.9 |
| Other indicationsc | 6 | 1.6 |
| Insomnia and other sleep initiation and maintenance disorders | 4 | 1.1 |
| Impulsiveness | 4 | 1.1 |
| Tinnitus | 4 | 1.1 |
| Substance addiction | 2 | 0.5 |
| Early- and late-onset ataxia | 2 | 0.5 |
| Dementia | 2 | 0.5 |
Concomitant drug data in patients receiving antiepileptic drugs are summarised in Table 1. Analgesics, anti-ulcer agents, antihistamines, and antidepressants were the most common drugs used in combination with antiepileptics in these patients.
Adherence, changes in prescription, and adverse effectsTable 1 displays data about treatment adherence and the number of patients who needed to change medications.
We recorded 31 different adverse effects associated with antiepileptic use in 23 patients (6.2%); 17 patients (4.6% of the patient total) had only one adverse reaction, 4 experienced 2 adverse reactions, and 2 experienced 3 unwanted effects. The antiepileptics responsible for adverse reactions were valproic acid in 9 patients (2.4%), carbamazepine and levetiracetam in 3 patients each, and gabapentin, lamotrigine, and pregabalin in 2 patients each. The most frequent associated adverse reactions were dyspepsia (n=7; 1.9%); somnolence (n=5; 1.3%); and weight changes, headache, elevated levels of liver transaminase enzymes, insomnia, and generalised rash in 2 patients each. In addition, each of the following symptoms was reported once: amnesia, nightmares, thrombocytopenia, dizziness, nausea, tremor, fatigue, and paraesthesia in the mouth.
Response to treatment and multivariate analysisThe number of patients not attaining seizure control in the last year is shown in Table 1. A total of 25 patients (14.4%) were considered to have drug-resistant epilepsy. In the subgroup of 200 patients receiving antiepileptics for reasons other than epilepsy, only 17 (8.5%) had been asymptomatic for one year or more, while the remaining patients still experienced symptoms of the disorder for which the drug had been prescribed.
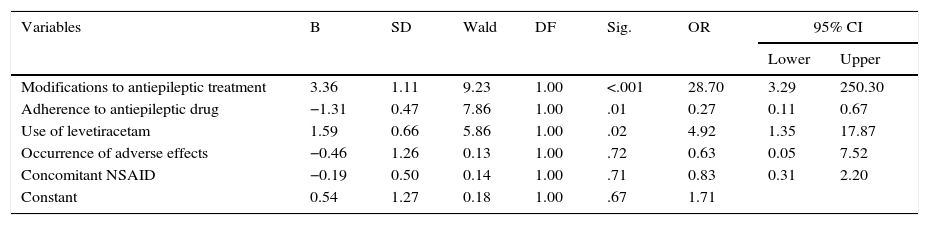
We used binary logistic regression to analyse the relationship between treatment failure and the variables significantly associated with the dependent variable in bivariate analysis, and found that modifying antiepileptic treatment and use of levetiracetam were associated with a greater risk of failure, whereas treatment adherence was associated with a lower risk of failure (Table 4).
Variables associated with treatment failure in epileptic patients, determined by a logistic regression model; Colombia, 2014.
| Variables | B | SD | Wald | DF | Sig. | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Modifications to antiepileptic treatment | 3.36 | 1.11 | 9.23 | 1.00 | <.001 | 28.70 | 3.29 | 250.30 |
| Adherence to antiepileptic drug | −1.31 | 0.47 | 7.86 | 1.00 | .01 | 0.27 | 0.11 | 0.67 |
| Use of levetiracetam | 1.59 | 0.66 | 5.86 | 1.00 | .02 | 4.92 | 1.35 | 17.87 |
| Occurrence of adverse effects | −0.46 | 1.26 | 0.13 | 1.00 | .72 | 0.63 | 0.05 | 7.52 |
| Concomitant NSAID | −0.19 | 0.50 | 0.14 | 1.00 | .71 | 0.83 | 0.31 | 2.20 |
| Constant | 0.54 | 1.27 | 0.18 | 1.00 | .67 | 1.71 | ||
B: regression coefficient; SD: standard deviation; DF: degree of freedom; 95% CI: 95% confidence interval; OR: odds ratio; Sig.: statistical significance.
The present study demonstrated the patterns of antiepileptic drug use in connection with the reason for prescription and other associated variables in a population of patients affiliated with the Colombian health and social security system. These findings may be used by doctors and healthcare providers to make decisions aimed at improving care for patients treated with antiepileptic drugs. We should stress that our data cannot be extrapolated to the Spanish population, not only because of sociodemographic factors and differences in medical training, but also considering the differences between our healthcare systems.
Mean age and sex ratio in our study population were similar to those previously reported in Colombia and such other countries as the United States, Italy, and the United Kingdom. However, some studies conducted in Taiwan have shown that antiepileptics are predominantly prescribed to men.8–13 Approximately 65% of the patients included in this study were adults, which is in line with previous studies.9,14
We have found no studies addressing prescription of antiepileptic drugs to treat both epilepsy and other conditions, and providing sociodemographic and anthropometric data. This is probably due to the design limitations of certain drug utilisation studies. In some studies of epileptic patients in Germany and Sweden, a smaller percentage of patients presents a low educational level, which contrasts with our results, a difference that can be expected if we compare Colombia with highly developed countries. Another study evaluating the effectiveness of pregabalin in patients with postoperative pain reported mean weight values similar to those found in our study.15–17
When comparing distribution by city with data from previous studies on drug utilisation in Colombia, we find obvious differences in the prescription patterns and frequency of use for some antiepileptics. This is not surprising since variability in medical care, especially in terms of drug prescription, is a common denominator in pharmacoepidemiology studies worldwide.8,15,18
In our study, we found that numerous patients had been prescribed conventional drugs (valproic acid, carbamazepine, and clonazepam), which contrasts with results reported in recent studies. According to the literature, use of conventional drugs has decreased in recent years while new drugs are on the rise due to their therapeutic potential, lower risk of adverse effects, and utility in conditions other than epilepsy. This may be explained by the fact that the Colombian healthcare plan features a list of specific drugs which must be prescribed before attempting treatment with new antiepileptics, even though patients do not have to pay for any of the drugs.9,17 Comparing our results to the prescription pattern for these drugs in Colombia in 2013 demonstrates a noticeable increase in the use of levetiracetam, which is consistent with the trend observed in other countries. The most likely explanation is that this drug was recently added to the Colombian healthcare service's drug list for patients with drug-resistant epilepsy. With this change in status, Colombian HIPs have no need for additional paperwork in order to dispense the drug.8,10,18,19
The doses of vigabatrin, lamotrigine, and oxcarbazepine used to treat some patients exceeded the recommended effective doses. Although it is true that doses may vary depending on the indication for prescription, in any case the probability of experiencing adverse effects increases with the dose.8,20 Interestingly, we found a high percentage of patients receiving phenobarbital, a drug that presents significant disadvantages compared to other less toxic antiepileptic agents.8,20
Our percentage of patients taking antiepileptic drugs in monotherapy is similar to rates reported in the literature (65.4% vs 61% and 82%). However, the number of patients receiving combination therapy is significant and may stem from failures to control symptoms or seizures with only one drug. In any scenario, the risk of adverse effects and drug interactions increases with combination therapy.10,13,14,21,22
Prescribing antiepileptics for indications other than epilepsy is an increasingly common practice, considering that only limited evidence is available about the drugs’ effectiveness for improving symptoms of tinnitus, hyperkinetic movement disorders such as ataxia, dementia, other types of non-neuropathic and rheumatic pain, and other disorders listed in retrospective non-randomised studies. The present study corroborates this hypothesis: 52.3% of patients received antiepileptics for non-epileptic conditions; this percentage is markedly higher than those reported by other authors.11,12,14,23,24
We found a high prevalence of painful conditions, acid peptic disease, and allergies among our patients, which resembles data reported by other authors.25,26 Nevertheless, prevalence of depression, hypertension, and dyslipidaemia was lower than in other studies conducted in Colombia, Germany, and Israel.8,18,27 We should consider potential interactions, since naproxen induces valproic acid metabolism, esomeprazole inhibits phenytoin metabolism, and fluoxetine and verapamil inhibit carbamazepine metabolism.21 In addition, tricyclic antidepressants may reduce the seizure threshold, which is not a desirable outcome for these patients.28
The percentage of non-adherence to antiepileptic treatment in our study (35.1%) is similar to those reported by other authors, which range between 32.0% and 63.0%. However, the percentage also depends on the drug.29,30 Some of the reasons for poor treatment adherence were adverse effects, not understanding the importance of adherence, polypharmacy, and age. Lack of adherence has severe consequences on both symptom control and quality of life.30,31
The percentage of patients whose treatment was changed was less than that reported in other studies in epileptic patients (13.9% vs 22-45%).22,31 Possible reasons are that different doses may have been used to treat non-epileptic disorders, which in turn may have affected the frequency of severe adverse effects and lack of adherence.
The small percentage of patients experiencing adverse effects compared to other studies (4.5% vs 8.7-88%) might simply be due to failure to record adverse effects in patients’ medical histories; adverse effects may have passed unnoticed or been considered mild or tolerable. The drugs causing adverse effects in our study population coincided with those reported in other studies, especially conventional antiepileptics, which are responsible for most of the adverse effects.22,32,33 However, the most frequently reported adverse effect in our study was dyspepsia, whereas nervous system alterations were predominant in other studies. The reasons for this discrepancy should be explored.6,32,33
The percentage of treatment failure (53.4%) was similar to those reported by some studies conducted in Scotland.34,35 In contrast, other studies have reported higher percentages of patients remaining seizure-free during at least one year.36 The percentage of patients with drug-resistant epilepsy resembled that in Europe (15.0%), although the patterns of antiepileptic drug use are different. This may be related to proper selection of the drug in both geographical areas or the inclusion of another active ingredient depending on the clinical presentation of epilepsy and the effectiveness of each of the drugs.37
Regarding the multivariate analysis, other studies have observed a connection between treatment adherence and pharmacological response in patients with epilepsy. Given the type of study, however, the association between levetiracetam and treatment modifications is likely due to decisions made by doctors in charge of treatment when seizure control had not been achieved.30,31
We should be mindful that the study methodology has several limitations, including the fact that data were gathered from medical histories, which means that adverse effects and lack of adherence may not have been recorded. In addition, our study population was composed of patients affiliated with the Colombian health and social security system; data may therefore be extrapolated to populations with similar access to medication and healthcare systems. Despite the above, the present study is relevant since the data search was conducted with great rigour and the study population constituted a representative sample of the population covered by paid insurers within the Colombian healthcare system (31.8%).
To the best of our knowledge, this is the first study in Colombia describing the reasons for prescribing antiepileptics. We can conclude that antiepileptics are used to treat both epilepsy and other distinct conditions. Monotherapy was the most widely used therapeutic approach in our sample, including prescription of conventional antiepileptics. In our sample population, adherence to antiepileptic drugs was associated with lower risk of treatment failure. Pharmacological response in patients with epilepsy was similar to that described for other populations.
In view of these findings, subsequent studies should aim to explore the effectiveness of these drugs for other indications and off-label use, and identify adverse effects, levels of adherence, and quality of life in patients treated with antiepileptic drugs.
Conflicts of interestThe study was financed by Universidad Tecnológica de Pereira and Audifarma S.A.
Please cite this article as: Machado-Alba JE, Calvo-Torres LF, García-Betancur S, Aguirre-Novoa A, Bañol-Giraldo AM. Estudio de prescripción-indicación en pacientes que reciben antiepilépticos en Colombia. Neurología. 2016;31:89–96.
This study has not been presented at any of the SEN's meetings.