Neurological manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being widely documented.1 However, de novo movement disorders are scantly reported in COVID-19.2 Apart from neurological manifestations, SARS-CoV-2 infection can make a previously euglycemic person vulnerable to develop either transient or permanent hyperglycemia.3,4
Osmotic demyelination syndrome (ODS), formerly called central pontine myelinolysis, is a serious neurological emergency, which arises from rapid changes in osmotic equilibrium in susceptible neuronal cells, especially oligodendrocytes.5 However, due to advancement in neuroimaging and our understanding of the pathophysiological processes underlying ODS, more cases of extra-pontine myelinolysis (EPM) are being reported.3 Among the complications of ODS, parkinsonism has been rarely reported.6,7
ODS occurs classically as a complication of the rapid correction of hyponatremia3; however, it has been rarely associated with other entities, such as hyperglycemic hyperosmolar state (HHS).8,9
We hereby report a case of an elderly diabetic woman with parkinsonism with akinetic mutism following non-dyselectrolytemic ODS, which was precipitated by COVID-19 induced HHS.
An elderly Indian woman aged 65 years with adequately controlled type-2 diabetes mellitus (T2DM) presented to the emergency department with fever, body-ache and dry cough for the last 5 days. She was diagnosed to have SARS-CoV-2 infection in the emergency department. Complete blood cell count revealed neutrophilic leukocytosis with neutrophil to lymphocyte ratio of 3; erythrocyte sedimentation rate, C-reactive protein, ferritin, lactate dehydrogenase and hepatic transaminases were raised. Serum electrolytes, blood glucose levels (pre and post-prandial), hemoglobin-A1c, ketones, d-dimer, interleukin-6, cardiac troponins, and thyroid and renal function tests were within normal range. A high-resolution computerized tomography scan of the thorax revealed a severity score of 8/25. She was prescribed inhalational budesonide, oral dexamethasone, doxycycline, ivermectin, antipyretics and subcutaneous basal-bolus insulin regimen. On day 4, she had bouts of unprovoked vomiting and became drowsy. Bedside random capillary blood glucose (CBG) measurement revealed high glucose levels (652mg/dl). Arterial blood gas analysis revealed no dyselectrolytemia or acid-base imbalances. Serum osmolarity was 317mOsm/kg with normal lactate levels. After 48h of stringent management and monitoring, she was still apathetic and responding rarely and incompletely to commands with long latency and became noticeably sluggish. A neurological examination revealed generalized bradykinesia, hypomimia, monotone and monosyllable incoherent speech, axial and symmetrical appendicular rigidity (MDS-UPDRS score of 60), and akinetic mutism. She had no other neurological deficits.
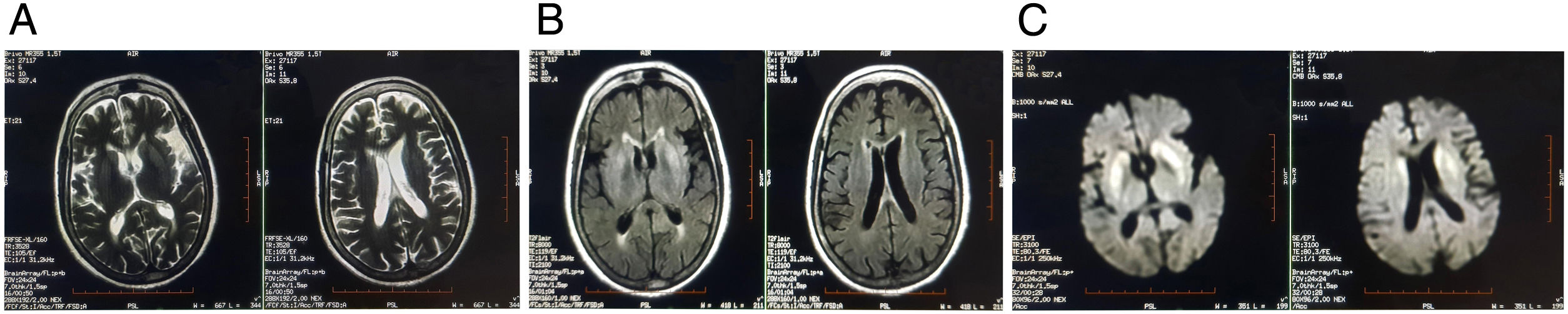
She had no history of addiction and no family history of any neurological disorders. Brain MRI revealed symmetrical hyperintense signals in T2-weighted imaging, T2-FLAIR and DWI over bilateral caudate nucleus and putamen (Fig. 1). Clinical–radiological differential diagnoses that were considered are shown in Table 1. Eventually, she was diagnosed to be a case of an extrapontine variant of osmotic demyelination syndrome (ODS) due to HHS, which was precipitated by COVID-19, that in turn had been treated with dexamethasone.
Clinical–radiological differential diagnoses of the case.
| Differential diagnoses | Odds in this case |
|---|---|
| Diabetic striatopathy | Should have resolved with swift and sustained control of blood glucoseNo corresponding T1-WI hyperintense signal changes over striatumUsually, diabetic striatopathy presents with hyperkinetic movement disorders |
| Sporadic Creutzfeldt-Jakob disease | No pyramidal featuresNo rapidly progressive dementiaHyperacute courseNo myoclonusNo visual cognitive deficitsNo cerebellar dysfunctionsEEG not suggestive of periodic sharp wave complexesNo typical cortical/gyral ribbon pattern or pulvinar sign on DWI |
| Metabolic encephalopathy | No improvement even with prompt correction of hyperglycemiaNever had any hypoglycemic episodeNo episode of dyselectrolytemiaHepatic functions including albumin, prothrombin time and international normalized ratio were normal. There was only mild elevation of levels of transaminase. Serum ammonia levels were normal.No myoclonus, tremor and asterixis were noted. |
| Hypoxemic encephalopathy | Patient was on continuous monitoring and never had an episode of hypoxemia/hypoxia.No myoclonus. |
| Wernicke's encephalopathy | Hyperacute courseNo ataxia, ophthalmoplegia and peripheral neuropathyNormal serum thiamine levelNo background risk factor for development of Wernicke's encephalopathyPeriaqueductal grey and mammillary bodies were sparedRepletion of thiamine had no effect |
| Autoimmune encephalitis involving basal ganglia (anti-D2, Anti-CRMP5) | CSF study was normalClinical history did not fit with the course of autoimmune encephalitisNegative antibody tests on CSF as well as serum for autoimmune encephalitis profile |
| COVID-19 induced encephalitis involving bilateral basal ganglia | Normal CSF parameters.No mass effect on neuroimaging.No other area of brain, e.g. thalamus, temporal/parietal lobes, inferior frontal gyrus, external capsule, etc. was involved. |
She was put on levodopa/carbidopa (initially started at 25/6.25mg 4 times a day and gradually up titrated to 100/25mg 5 times a day) and pramipexole (1.5mg/day). After 2 months of follow-up, her features of parkinsonism improved significantly (MDS-UPDRS score of 60 to 20), but with only mild improvement of the features associated with akinetic mutism. Pramipexole was up titrated to 3mg/day and sertraline (100mg/day) was added. Throughout her hospital stay, her minimum serum sodium (corrected for hyperglycemia) was 136mEq/L and maximum was 142mEq/L. The change in serum sodium was closely monitored and never crossed >5mEq/L. After another 2 months of follow-up, her mutism also improved significantly and she is currently continuing the same regimen along with drugs and lifestyle modifications for T2DM.
We have described a case of parkinsonism with akinetic mutism associated with the worsening of glycemic control in an elderly diabetic woman suffering from SARS-CoV-2 infection. An event-by-event discussion will aid us in dissecting the pathophysiology of this case.
Firstly, the loss of glycemic control might either be because of corticosteroid therapy, COVID-19 infection itself or lack of physical activity.3,4
Secondly, the ODS results from maladaptive stress due to rapid change in the osmotic milieu within the neurons, possibly due to disruption of blood–brain barrier, exposure of glial cells to activated complements and cytokines, which in combination eventually lead to axonal shear injury, cellular energy depletion and apoptosis.5 However, some researchers attribute this to an osmotic disequilibrium-related endothelial damage and the subsequent release of inflammatory mediators from the injured endothelial cells.8,10 Rapidly developing HHS is a rare, although documented, risk factor of ODS.8,9 It is highly plausible that in our patient, HHS developed rapidly enough that oligodendrocytes could not adapt. Interestingly, all the previously mentioned pathogenetic mechanisms (i.e. endothelial damage, disruption of blood–brain barrier, cytokine-mediated cellular injury, etc.) leading to ODS can also occur in COVID-19 itself,11,12 and this raises the question whether the SARS-CoV-2 infection itself is capable of causing ODS. However, we feel that in our patient was due to the rapid surge in plasma glucose and thereby plasma osmolarity.
Thirdly, what caused parkinsonism with akinetic mutism? There are three possibilities. The uncontrolled hyperglycemic state is established as a cause of potentially reversible de novo movement disorders (diabetic striatopathy).13,14 Besides, the EPM variant of ODS is particularly known for its predilection to give rise to de novo movement disorders because of the involvement of the crucial striato-thalamo-cortical networks.6,7 Furthermore, SARS-CoV-2 infection itself can give rise to this type of movement disorder.2
Finally, the question is whether the neuroimaging findings can be the result of COVID-19 encephalitis/encephalopathy or autoimmune basal ganglia-encephalitis, triggered by SARS-CoV-2 infection. Basal ganglia involvement in COVID-19 encephalitis and COVID-19 related autoimmune encephalitis have been documented.2,15 Normal CSF study and negative autoimmune encephalitis panel in both CSF and serum pointed towards the diagnosis of ODS over viral/immune encephalitis. Besides, the onset of the disease with acute rapid deterioration of blood glucose control and the HHS further strengthened the argument in favor of ODS.
In closing, both COVID-19 and dexamethasone worsened the glycemic control of this previously well-controlled diabetic elderly woman further leading to the development of non-dyselectrolytemic ODS. This ODS alone or perhaps, in conjunction with SARS-CoV-2 infection itself, led to akinetic mutism and parkinsonism, which responded to treatment.
FundingThis study was not funded.
Author contributionsAll authors contributed significantly to the creation of this manuscript; each fulfilled criteria as established by the ICMJE.
Conflict of interestThe authors declare that they have no conflict of interest.