The scope of this study was to assess salivary and serum osteoprotegerin (OPG) levels in knee osteoarthritis (OA).
MethodsSerum and saliva OPG levels of 30 knee OA and 30 matched healthy controls in this cross-sectional study was assessed by ELISA. Knee pain was assessed by WOMAC. Data were analyzed by Student's t-test, Spearman correlation test and ROC.
ResultsThe mean serum but not saliva OPG level was lower in knee OA than that of the healthy group. WOMAC negatively correlated with serum OPG (r=−0.501; P=0.000). The serum OPG cutoff value was 237.5pg/ml for the diagnosis of knee OA.
ConclusionsAs serum OPG was lower in knee OA and negatively correlated with WOMAC, it seems that detection of OPG in serum but not in saliva may be a probable marker to the diagnosis of knee OA.
Key messagesOsteoprotegerin decreases in knee osteoarthritis.
El alcance de este estudio fue evaluar los niveles de osteoprotegerina (OPG) salival y sérica en la osteoartritis de rodilla (OA).
MétodosLos niveles de OPG en suero y saliva de 30OA y 30 controles sanos emparejados en este estudio transversal se evaluaron mediante ELISA. El dolor de rodilla fue evaluado por WOMAC. Los datos se analizaron mediante la prueba t de Student, la prueba de correlación de Spearman y ROC.
ResultadosEl nivel medio de OPG en suero, pero no en saliva fue menor en la artrosis de rodilla que en el grupo sano. WOMAC se correlacionó negativamente con la OPG sérica (r=−0,501; p=0,000). El valor de corte de OPG sérico fue de 237,5pg/m1 para el diagnóstico de OA de rodilla.
ConclusionesComo la OPG sérica fue más baja en la artrosis de rodilla y se correlacionó negativamente con WOMAC, parece que la detección de OPG en suero, pero no en la saliva puede ser un marcador probable para el diagnóstico de artrosis de rodilla.
Mensajes claveLa osteoprotegerina disminuye en la osteoartritis de rodilla.
Osteoarthritis (OA) or degenerative joint disease is one of the most common chronic degenerative diseases and is one of the important causes of pain and inability worldwide and is associated with increased treatment costs, decreased productivity, and absence from work.1–3 The prevalence of knee OA is higher among patients with OA.4 The incidence of OA is increasing because of population aging and overweightness 5and it is assessed that 10% of men and 18% of women over 60 years of age have OA.6 Accordingly, OA has a noteworthy negative impact on the quality of life of the old and the unseen budgets are high, so it put on a substantial burden on the health care system.6
Osteoprotegerin (OPG), as a controller of osteoclasts differentiation and function, is a growth factor receptor and depends to the tumor necrosis factor receptor family that contacts to receptor activator of nuclear factor-kB ligand (RANKL) subsequently inhibits connection of RANKL to receptor activator of nuclear factor-kB (RANK) and thus have a protecting effect versus bone demolition.7 Comparable osteoblasts, chondrocytes make OPG and RANKL. It has been shown that changes in RANK, RANKL, and OPG levels are linked to basic abnormalities of the joint cartilage in OA.8 Also, overexpressed of RANKL has been shown in OA.9
OA is today recognized based on radiographic norms and clinical signs.10 Magnetic resonance imaging (MRI) is a non-invasive technology that can provide images of structural changes in all joint constructions.11 However, this technique is a time consuming and costly procedure. Earlier studies have recommended certain biomarkers can be used in the diagnosis or the progression of OA. The bioindicator that has been precisely demarcated for OA can be associated with bone, cartilage, and synovium metabolisms or systemic irritation.12
It has been shown that saliva as an indicator of oral and systemic health, can offer dependable evidence about the disease. Non-invasive matter, easier gathering and more cost-effective of saliva equated to serum and synovial fluid (SF) has prompted researchers to study about saliva.13,14 It has been described that serum levels of OPG increases in patients with OA.15,16 Therefore, in this study, OPG was evaluated in serum and saliva samples of patients with knee OA to evaluate the potential of this marker in the diagnosis and monitoring of the disease.
MethodsIn a cross-sectional study, based on the American College of Rheumatology, radiological and clinical criteria for OA of the knee system,17 30 patients with a grade 2 or 3 knee OA based on the Kellgren and Lawrence (KL) classification18 (19 male/11 female; aged 55.3±3.4 years) who candidate for stem cell therapy of knee joint in Firoozgar Hospital and had no history of gout, knee surgery, joint trauma, and rheumatic disease and had no treatment might interfere with bone metabolisms, and also 30 sex and age-matched healthy individuals (19 male/11 female; aged 54.5±3.2 years) were enrolled in this study. The healthy participants had no sign of symptomatic and radiological osteoarthritis as evaluated by the clinical examination of the knees by a skilled orthopedist. All of them were healthy without any disease or treatment that might affect bone or joint metabolism, including hormone replacement therapy in postmenopausal women.
This study was approved by the Review Board of the National Institute for Medical Research Development (IR.NIMAD.REC.1396.206) and informed written consent was obtained from all participants.
The Patients’ symptoms and physical disabilities were evaluated using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index.19 They are each divided on a 5-point Likert scale (none, mild, moderate, severe, and excessive). The WOMAC score ranged between 0 and 96. Patients filled in the WOMAC Persian language questionaries before cell therapy.
Serum and saliva were accomplished from each participant in the morning. Serum and saliva samples were collected as previously described.13 The specimens were centrifuged at 5000g for 10min; the serum and saliva supernatants were stored at −80°C for later measurement of OPG. OPG was assessed by the sandwich ELISA method based on the manufacturer's protocol (BioAssay Technology Laboratory, Shanghai, China). The sensitivity of the ELISA OPG kit was 23pg/ml (0.023ng/ml).
The data are offered as a mean±SEM. Assessment of means amongst groups was performed with an unpaired two-tailed student's t-test. The Spearman correlation test was used to confirm the association between the parameters. Receiver operating characteristic (ROC) analysis was used to detect cut-off point for serum and salivary OPG between OA and healthy groups. Analyses were done using SPSS software version 16.
ResultsThere was no significant difference in sex and age between healthy and OA groups (P<0.05). The mean (±SD) WOMAC score of OA group was 37.7±4.9. There was no significant difference in BMI (mean±SEM, kg/m2) between healthy (28.1±0.5) and knee OA (28.4±0.7) groups (P=0.636).
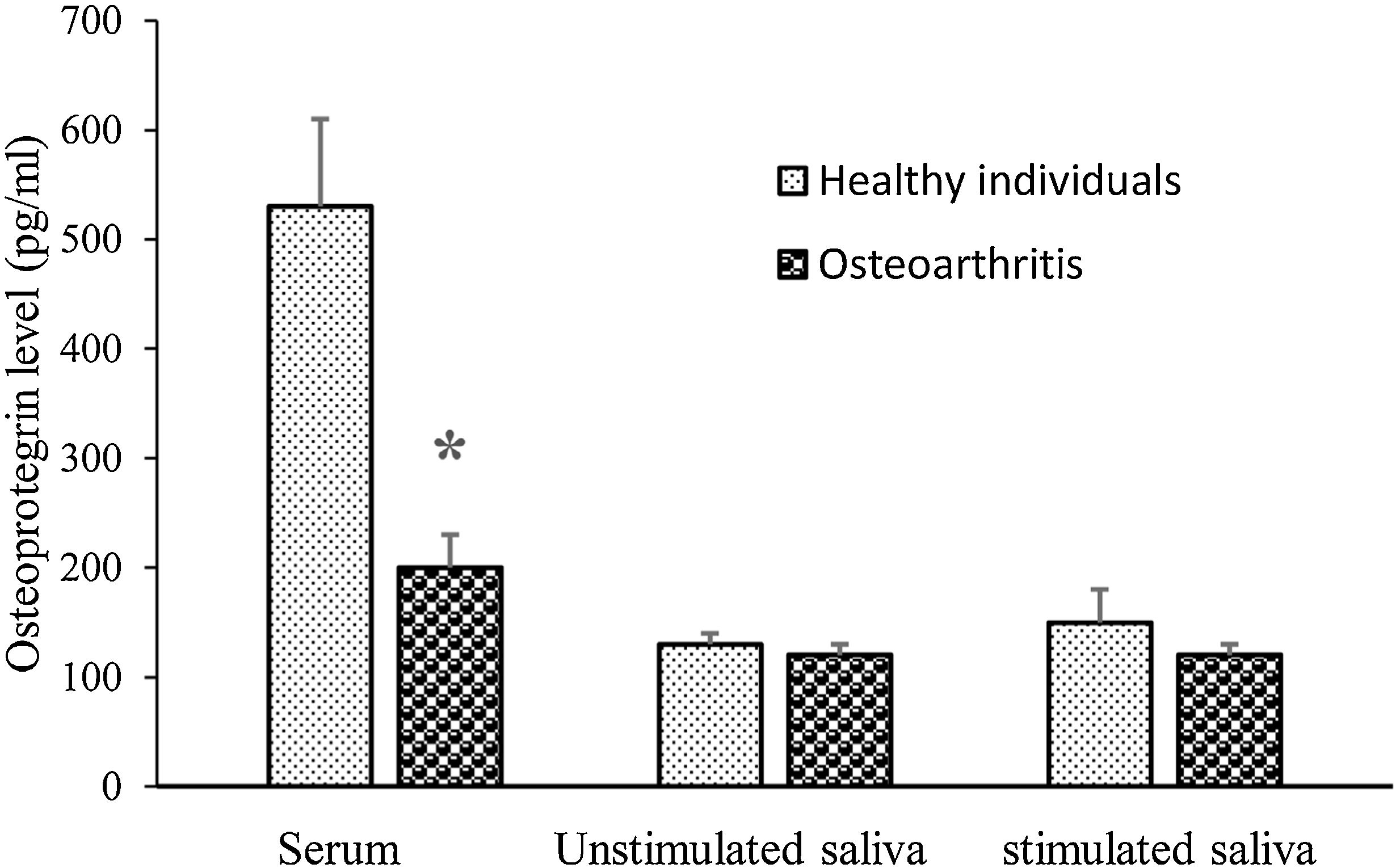
The mean serum OPG concentration was lower in OA than that of the healthy group (P=0.001) (Fig. 1). However, stimulated and unstimulated salivary concentrations of OPG were not significantly different between OA and control group (P=0.317, P=0.712; respectively).
WOMAC score negatively correlates with serum OPG level (r=−0.501; P=0.000). However, there was no significant correlation between WOMAC score and stimulated saliva OPG level (r=−0.097; P=0.361) and also with OPG unstimulated saliva (r=−0.102; P=0.470).
The serum level of OPG was not significantly correlated with unstimulated (r=0.186; P=0.188) and stimulated (r=0.134; P=0.366) saliva OPG levels.
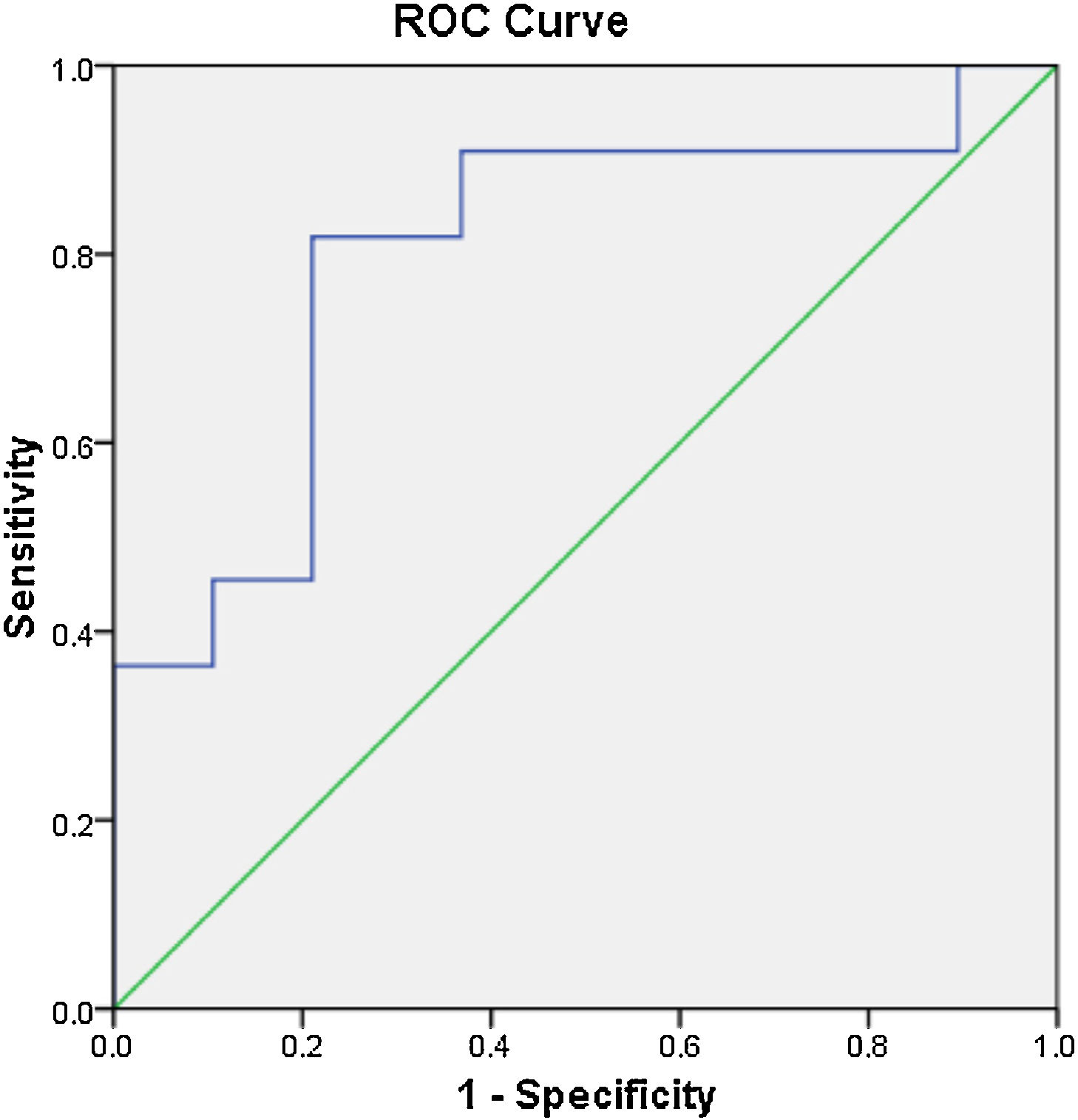
The ROC analysis results revealed that the cut-off value of serum OPG for the diagnosis of OA was 237.5pg/m1 (ROC-area under the curve=0.799). With this cut-off, sensitivity was 82%, and specificity was 79% (Fig. 2).
DiscussionToday, biomarkers, which are applied to evaluate the activity of disease or to screen treatment effectivity in OA depend on MRI outcomes. Relative invasiveness resulting from blood or SF collection is another cause for a need for new simple, cost-effective and non-invasive biomarkers. Saliva can be collected noninvasively, with easy access and the possibility of repeated sampling. Therefore, the saliva represents an attractive diagnostic fluid for distinguishing biomarkers of various pathological conditions.20–28 In OA patients, there was a little study about markers in other biofluids apart from plasma and SF. Also, the possibility of measuring these in saliva brings non-invasive techniques and therefore more compliance from patients. This study aimed to analyze OPG in serum and saliva samples of patients with OA. Our results showed that serum OPG was lower in knee OA and negatively correlated with WOMAC. However, stimulated and unstimulated saliva OPG were not differ between two groups and did not correlate with WOMAC.
OA is a degenerative joint disease categorized by the slow destruction of the cartilage matrix and by bone changes.29 It has been shown that mice lacking both OPG alleles have the more severe degenerative joint disease and administration of OPG in OA mice protects articular cartilage destruction.30,31 Also, it has been indicated that OPG-deficient mice exhibit thin articular cartilage layers,31 show hard destruction of growth plate cartilage and show some degree of cartilage destruction with aging.30,31 They agree with our results that serum OPG level was lower in patients with OA than in healthy individuals and the serum OPG concentration negatively correlated with WOMAC as an indicator of knee OA severity in this study. However, our result is in contrary to Min's et al.15and Pilichou et al.16 reports that showed serum OPG level is higher in OA than healthy individuals. The majority of the participants in the mentioned studies were female (female/male≈4), but most were male (female/male≈0.6) in our study. Perhaps this paradox is related to gender which needs further study to prove it.
OPG is a soluble receptor that binds to RANKL and inhibits binding of RANKL to RANK which presents on the membrane of preosteoclast,32 consequently inhibits osteoclastogenesis.33 RANKL mRNA expression has been shown to increase in the cartilage of patients with grade 2 OA.34 RANKL also increases MMP-13 expression in these patients. Studies have shown that overexpression of MMP-13 plays a key role in the destruction of articular cartilage and morphological changes in bone tissue in OA.35 The RANKL in the deep cartilage region may be distributed from a thin layer of the calcified cartilage into the bone where it can bind to RANK in osteoclastic precursors in the subchondral bone. Also, it has been indicated that RANKL increases in the serum of patients with knee OA.16 It seems that OPG production reduces and RANKL production increases in knee OA, so MMP-13 overexpressed in these patients which cause cartilage and bone destruction in joints of these patients. In addition, it has been shown that cortisol is higher in OA and associates with WOMAC and glucocorticoids reduce serum OPG.36,37 It may explain serum OPG reduction in the OA patients.
WOMAC negatively correlated with serum OPG in this study. The ROC analysis indicated a cut-off value of 237.5pg/m1 for serum in the differential diagnosis of OA and normal individuals. These results suggest that OPG may be related to the pathogenesis of OA and is a potential biomarker for OA.
There were no significant differences in saliva OPG between knee OA and healthy individuals. To our best knowledge, this is the first study about the detection of OPG in the saliva of OA patients. It seems that detection of OPG in the saliva is not a potential biomarker for OA.
There were some limitations for this study. As patients with knee OA in grade 2 and 3 were enrolled, we cannot evaluate the association of OPG with the radiological degree of the knee OA. In addition, for accurate calculation of the cutoff point of serum OPG, the sample size was low.
ConclusionOur results showed that as serum OPG was lower in knee OA and negatively correlated with WOMAC, it seems that detection of OPG in serum but not in saliva may be a probable marker to the diagnosis of knee OA.
Level of evidence II.
Ethical approvalThis research was approved by the Ethics Committee of the National Institute for Medical Research Development (NIMAD) with the approval code IR.NIMAD.REC.1396.206.
Funding/supportThe research reported in this publication was supported by Elite Researcher Grant Committee under award number 963466 from the National Institute for Medical Research Development (NIMAD), Deputy of Research and Technology, Ministry of Health and Medical Education of Iran.
Conflict of interestThe authors declare that there is no conflict of interests.