Patients from other centres where they have had an unplanned surgical resection of a soft tissue sarcoma are often referred to hospitals specialised in sarcomas.
Material and methodsA study was conducted on 35 patients who required this type of surgery were referred to our centre between November 2001 and July 2013.
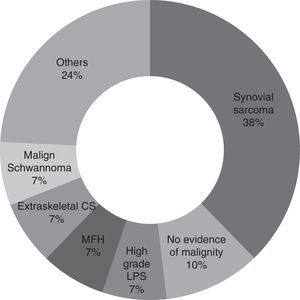
ResultsSurgery had been performed on 29% of the patients without any complementary tests being done. In 75% of cases, the sarcoma diagnosis was discovered in the post-surgical histological study. Synovial sarcoma was the most common, affecting 38% of the patients.
A surgical revision of the margins was performed on all of them, and adjuvant treatment was performed on 86% of them. The histopathology study found that 69% of the patients had residual disease.
At the end of follow-up, 12% had a local recurrence, another 12% distant metastases, and 3% had died.
ConclusionGiven the results, it is concluded that any tumour of the soft tissues in which malignancy is suspected has to be resected in a reference centre. If an unplanned esection was performed in another centre, it should be referred immediately in order to perform an imaging study, revision surgery, and if required, adjuvant treatment.
En ocasiones, a los hospitales especializados en sarcomas son derivados pacientes desde otros centros donde se les ha realizado una cirugía no planeada de resección de sarcoma de partes blandas.
Material y métodosEntre noviembre del 2001 y julio del 2013, 35 pacientes de este tipo fueron derivados a nuestro centro.
ResultadosEl 29% de los pacientes había sido intervenido sin pruebas complementarias previas. En el 76% el diagnóstico de sarcoma se descubrió en el estudio histológico postoperatorio. El sarcoma sinovial fue el más común encontrado, afectando el 38% de los pacientes.
A todos ellos se les realizó cirugía de revisión de márgenes y en el 86% se realizó terapia adyuvante. El 69% de los pacientes tenían enfermedad residual hallada en la anatomía patológica.
Al final del seguimiento, el 12% había presentado recidiva local, otro 12% diseminación a distancia y el 3% había fallecido.
ConclusiónDados los resultados, concluimos que cualquier tumoración de partes blandas de la que se sospeche malignidad ha de ser resecada en un centro de referencia, y si se ha producido una resección no planeada en otro centro tiene que ser derivado inmediatamente para la realización reglada de un estudio de imagen, cirugía de revisión y, si precisa, tratamiento adyuvante.
Sarcomas are malign tumours which occur in tissue of mesenchymal origin, so that they may appear in bones as well as in soft tissues. Although they make up less than 1% of all malign tumours, they cause great morbidity and mortality at any age and in any location. Many of them present as a painless swelling which gradually grows in size.1,2
Given that soft tissue sarcomas are not very common and are far less frequent than benign tumours, it is possible that some of them go unnoticed, and that it would be inappropriate to resect them before establishing the correct histological diagnosis. It is calculated that centres specialising in sarcomas receive from 19% to 53% of patients referred from other hospitals after unplanned surgery. The risk of residual disease after unplanned resection stands at 24–60%.3 Tumour relapse is 2.2 times more common in these patients than it is in those who had received appropriate study prior to surgery.1,2
To manage these patients the specialised centre has to gather the maximum amount of information about the case. The data supplied by the patient as well as the surgeon who first operated are therefore highly important. These include the results of any preoperative or postoperative study and the details of the surgery. It is also important to revise the histological samples from the tumour, not only to confirm the diagnosis, but also to discover the surgical margins, given that these are predictors of the risk of local relapse.4 The therapeutic options implemented in the reference centre often run from abstaining from therapy with regular checks on the patient, to revision surgery of the margins (“second-look surgery”), as well as the isolated or concomitant use of adjuvant therapies (radiotherapy and/or chemotherapy).1
This study aims to describe the oncological evolution of a group of patients who were subjected to revision surgery of soft tissue sarcoma margins after they had previously been subjected to unplanned surgery for the resection of the same.
Material and methodsWe carried out a descriptive longitudinal observational and retrospective study of the relevant data in our database of patients with muscle or bone tumours. We included all of the patients aged 18 years old or above who had been sent to the orthopaedic oncology unit in our hospital after resection with positive margins of a soft tissue sarcoma located in the upper or lower limbs.
Firstly we gathered patient epidemiological data as well as data relating to certain aspects of the disease, such as location or the first symptom. We also recorded information about the first operation, such as additional testing beforehand (MRI, computerised tomography [CT], ultrasound scans and biopsies, etc.), the diagnosis of suspicion prior to surgery, the time lapse until surgery, the type of surgery (broad surgery or simple resection) and the result of the pathologic study of the samples obtained during the same. For the margins revision or second-look surgery we collected similar data, including the pathological diagnosis of the revision in our hospital of the histological sections from the first operation, imaging studies prior to the revision surgery, the time elapsed between the first and revision operations, and the classification of the residual disease based on the classification of the American Joint Commission on Cancer.5 Finally, we gathered information about adjuvant treatment and the clinical evolution of patients.
ResultsFrom November 2001 to July 2013 35 patients who had been subjected to an unplanned resection of a soft tissue sarcoma were treated in our unit. They all fulfilled the inclusion criteria described. 18 of these patients were women (51.4%) and 17 were men (48.6%). Their average age was 48 years old (range from 17 to 29).
The most common location of the disease was the thigh (42.9% of cases, 15 cases), followed by the foot (14.3%, 5 cases), the leg (8.6%, 3 cases), the groin (8.6%, 3 cases), at shoulder blade level (5.7%, 2 cases) and the knee (5.7%, 2 cases). The other cases were in the arm, elbow, hand, pelvic waist and the ankle (with one case in each location, 2.9%); 14 cases (40%) were in the right side and 21 cases (60%) were in the left side.
The average duration of patient follow-up was 52 months (range from 5 to 128 months).
The first symptom described by patients was the appearance of a painless swelling (85.7%, 30 cases), followed by a painful swelling (11.4%, 4 cases). Only one case (2.9%) presented isolated pain.
97.1% of the patients (34 cases) were referred to the oncological orthopaedic unit from another centre. The remaining 2.9% (one case) were referred from the General Surgery department of our hospital with the definitive diagnosis of leiomyosarcoma in what had originally been suspected to be a groin adenopathy.
76.4% (26 cases) of the patients from other centres were referred following a surprise diagnosis in the analysis of the intraoperative pathological samples obtained, which is known as “whoops surgery”. 17.6% (6 cases) were referred after positive tumour margins were found. The remaining 5.9% (2 cases) were referred when the tumour was found to persist by complementary imaging tests after surgery.
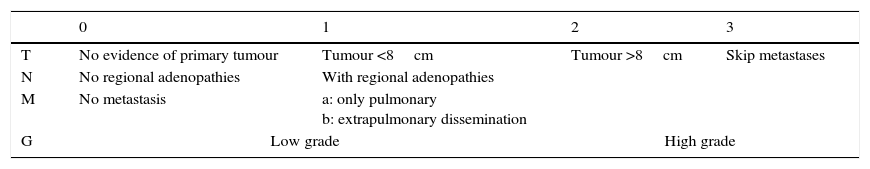
54.3% of lesions were at the level of the fascia (19 patients), while in 45.7% (16 patients) they were superficial. The resected tumour was less than 8cm in size in 91.4% of the patients (32 cases), i.e., T1 according to the American Joint Commission on Cancer (AJCC) staging system (Table 1). The 3 remaining cases (8.6%) correspond to T2 tumours according to the same staging system.
American Joint Commission on cancer staging.
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| T | No evidence of primary tumour | Tumour <8cm | Tumour >8cm | Skip metastases |
| N | No regional adenopathies | With regional adenopathies | ||
| M | No metastasis | a: only pulmonary b: extrapulmonary dissemination | ||
| G | Low grade | High grade | ||
Grades: I-A: T1, N0, M0, G1-2; I-B: T2-3, N0, M0, G1-2; II-A: T1, N0, M0, G3-4; II-B: T2, N0, M0, G3-4; III: T3, N0, M0, G3-4; IV-A: T1-3, N0, M1a, G0-3; IV-B: T1-3, N1, M0-1, G0-3/T1-3, N0-1, M1b, G0-3.
Only one case (2.9%) presented close tumoral adenopathies, while only one case (2.9%) presented pulmonary metastasis in the diagnosis.
The most common degree of tumour according to the AJCC classification was therefore II-A in 22 patients (62.9%), followed by II-B (5 cases, 14.3% of cases), I-B (2 cases, 5.7% of cases) and II-B, IV-A and IV-B (with one case each, 2.9% of cases each).
The initial surgery had taken place without complementary tests in 10 cases (28.6%), while in 16 cases (45.7%) only one complementary test had been performed. Only 5 patients (14.3%) were subjected to biopsy beforehand, and 2 patients (5.7%) received fine needle aspiration biopsy (FNAB). A MRI technique was performed in 45.7% of cases (16 cases). Other imaging tests used were ultrasound scans (20%, 7 cases), CT (11.4%, 4 cases) and simple X-ray (5.7%, 2 cases).
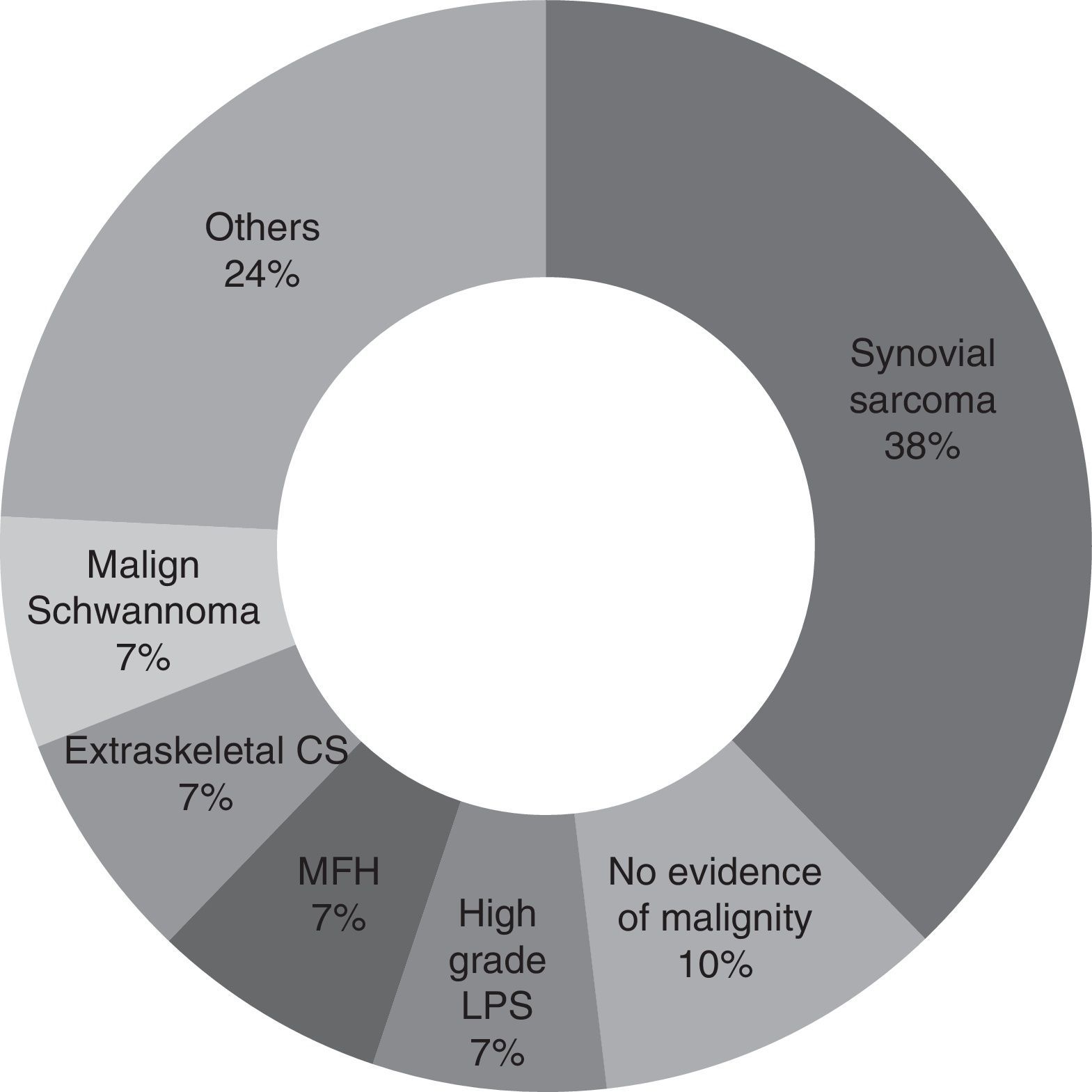
For the majority of patients (82.9% of cases, 29 patients) the samples obtained in the first operation were revised again by pathologists in our hospital with experience in tumours of this type. Samples were not revised in the other cases because of bureaucratic difficulties in obtaining the pathological sample sections. It may therefore be concluded that the most common diagnosis is synovial sarcoma (37.9%, 11 patients), followed by malign fibrohistiocytoma, high grade liposarcoma, extraskeletal condrosarcoma and malign schwannoma (6.9%, 2 patients each). Other diagnoses were pleomorphic sarcoma, extraskeletal osteosarcoma, leiomyosarcoma, fibrosarcoma, mesenchymal sarcoma, clear cell sarcoma and atypical fusocellular proliferation (3.4% and one patient each) (Fig. 1).
When patients arrived at our hospital a new MRI test was performed before revision surgery in 91.4% of cases (32 patients). 53.1% of these patients presented a residual tumour according to this complementary test (17 patients).
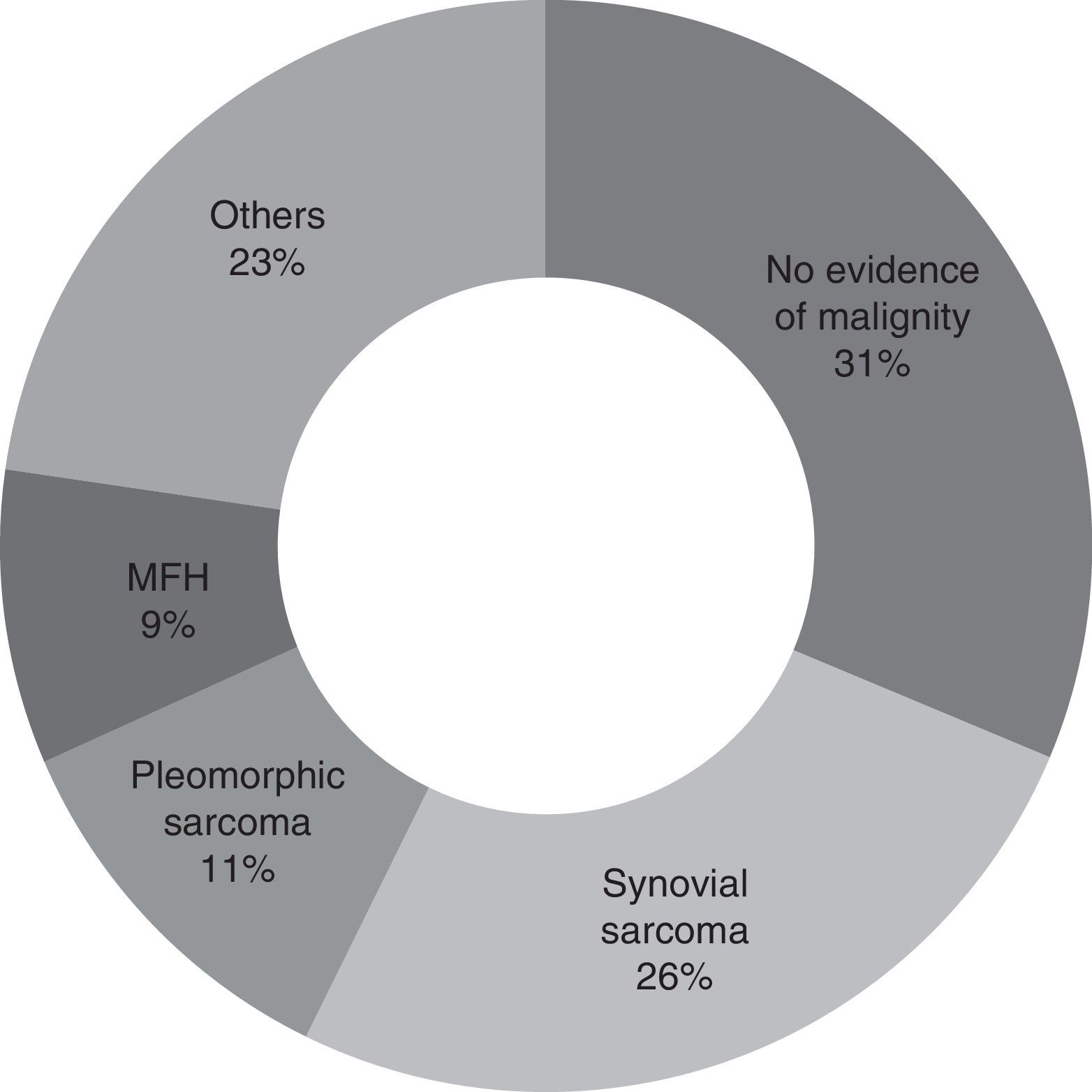
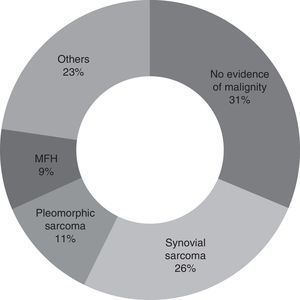
Second look surgery was undertaken from 1 to 9 months after the first operation (with an average of 3 months). The most common diagnosis found was synovial sarcoma, in 25.7% of the patients (9 patients). In 31.4% of the patients (11 cases) no evidence of malignity was found. A total of 8 patients (22.8%) had positive tumour margins after resection (Fig. 2).
More than half of the patients (51.4%, 18 patients) also received adjuvant chemotherapy and radiotherapy. 25.7% (9 patients) only received radiotherapy after revision of the margins, while 8.6% (3 patients) only received adjuvant chemotherapy prior to the second look.
At the end of follow-up a total of 4 patients (11.4%) had a local relapse. Two of them had positive margins in revision surgery histology. 4 other patients (11.4%) had systemic dissemination. One patient (2.9%) died due to pulmonary progression.
DiscussionThe concept of the complete unplanned resection of soft tissue sarcomas was introduced for the first time by Giuliano and Eilber.6 It refers to the situation in which no previous type of diagnosis has taken place prior to surgery, which is performed without the intention of achieving free tumour margins.7
Given that there are a higher proportion of benign tumours in the soft tissues than malign ones, sometimes malign tumours may be resected in unplanned surgery. Several series in the literature show a significant number of patients were operated for tumours which unexpectedly proved to be malign.1,6–16 This may be due to a lack of knowledge about the management of soft tissue sarcomas and this may be problematic if their potential malignity is not considered.14,15
To prevent the unplanned resection of soft tissue sarcomas a number of signs which may indicate malignity have to be taken into account. These include indurate consistency, a tumour size >5cm, a rapid increase in size, the presence of the mass at the depth of the fascia, pain and recurrent swelling.14,16 If there is a suspicion of malignity the relevant tests must be performed prior to the operation, including biopsy and imaging studies.3 In our series the initial surgery took place without previous complementary tests in 28.6% of cases. A biopsy was taken beforehand in only 14.3% of cases, while a MRI study was performed in 45.7% of cases. We found no similar data in the literature with which our results could be compared.
Synovial sarcoma was the tumour found the most often in unplanned surgery (37.9%) in our series. This agrees with the results obtained by Chandrasekar et al.,1 although in their case this type of tumour represents 17% of the cases in their series. The most common tumour types found by Lewis et al.10 in their series were malign fibrohistiocytoma (MFH) and liposarcoma (LPS), at 28% and 26%, respectively. In the series of Fiore et al.13 they found the most common malign tumour to be liposarcoma, in 36% of cases. In our study malign fibrohistiocytoma and high grade liposarcoma are in second place, each with 6.9% of cases.
The typical lesion found in our series would therefore be a synovial sarcoma smaller than 8cm and located in the thigh. The typical patient, without adenopathies or metastasis, would be in AJCC stage II-A.
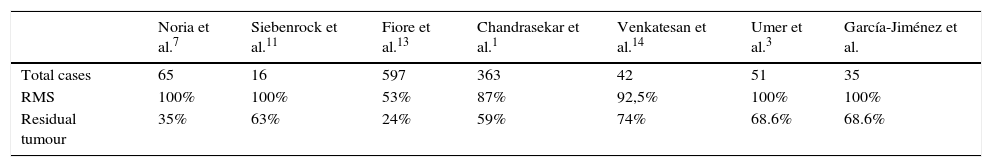
As was observed in previous studies,1,6–10,12,13 the unplanned resection of a soft tissue sarcoma involves the incomplete cutting out of the same in the majority of cases (Table 2). The incidence of residual tumour being found in revision margins surgery is similar to that in series such as that of Chandrasekar et al.,1 which stands at 60%. In our series we found residual tumour in 68.6% of patients (24 cases), although the actual figure is probably slightly higher, given that one patient (2.9%) without the finding of residual tumour in revision margin surgery went on to suffer a local relapse. We can therefore estimate that, in our series 71.4% of the patients subjected to an unplanned operation had residual tumour in the surgical site. It is due to this high number of patients with residual tumour after the unplanned resection of a soft tissue sarcoma that in our centre we routinely perform revision surgery of the margins in such cases.
Comparison of the number of cases with residual tumours in several studies.
| Noria et al.7 | Siebenrock et al.11 | Fiore et al.13 | Chandrasekar et al.1 | Venkatesan et al.14 | Umer et al.3 | García-Jiménez et al. | |
|---|---|---|---|---|---|---|---|
| Total cases | 65 | 16 | 597 | 363 | 42 | 51 | 35 |
| RMS | 100% | 100% | 53% | 87% | 92,5% | 100% | 100% |
| Residual tumour | 35% | 63% | 24% | 59% | 74% | 68.6% | 68.6% |
RMS: revision margin surgery.
Revision surgery has to include broader margins than those which would have been included in planned surgery for the resection of a soft tissue sarcoma. Imaging studies (CT or MRI) may be negative as they emphasise postoperative changes that prevent the correct visualisation of residual disease, while habitually unsuitable surgical incisions and approaches compromise and contaminate compartments adjacent to the tumour.3,17
The value of adjuvant therapies following sarcoma margin revision surgery is unclear.1 Chemotherapy, which was used alone in 8.6% of our patients and in 51.4% together with radiotherapy, plays a complementary role to margin revision surgery. Radiotherapy, which was used alone in 25.7% of our patients, is indicated routinely in many hospitals following the resection of a tumour.10,13
According to the literature, local relapse and metastasis are greater in patients who receive second look surgery.1,10,14,18 The incidence of local relapse in our series was 11.4%. This is comparable to the rates observed in other series,1,6–10,12,13 in which the incidence stands at from 7% to 26%. In 50% of the patients who presented a relapse (2 patients, 5.7% of the total) margin positivity had been observed in the histology of the samples obtained during revision surgery in our hospital. 11.4% of the patients presented remote dissemination, a lower figure than that found in other series.1
The overall survival rate of the patients in our series was 97.1% at the end of follow-up. This survival rate is higher than those in other series, which stand at 77–88%.1,10,13
The results obtained in our series show no major differences from those of other published series in hospitals in other countries. We can conclude that the high rate of residual tumour identified in our series makes it recommendable to resect any soft tissue tumour in a sarcoma reference hospital.19,20 In those cases in which the tumour was resected in an unplanned operation in another hospital, it is recommendable to refer the case to a reference hospital where a new revision of histology and MRI studies will be undertaken to detect residual disease, together with revision surgery of the tumour bed.
This is why in our unit we currently systematically recommend broad revision of the surgical site following the unplanned resection of a sarcoma.
Level of evidenceLevel of evidence iii.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments in human beings or animals were performed for this research.
Data confidentialityThe authors declare that they followed the protocols of their centre of work governing the publication of patient data.
Right to privacy and informed consentThe authors declare that this paper contains no patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García-Jiménez A, Trullols-Tarragó L, Peiró-Ibáñez A, Gracia-Alegría I. Análisis de resultados en cirugía de revisión de márgenes de sarcomas de partes blandas. Rev Esp Cir Ortop Traumatol. 2016;60:366–371.