For successful anterior cruciate ligament (ACL) reconstruction, revascularisation and histological maturation are necessary, as their failure can cause graft rupture.
PurposeThe purpose of this study was to describe differences in the histological maturation of early failed plasty (less than 12 months after surgery) and late failed plasty (more than 12 months after surgery) in patients with re-rupture after ACL reconstruction with hamstring tendons.
Material and methodsA descriptive observational study was conducted on a consecutive series of 20 patients whose ACL reconstruction had failed. Graft biopsy samples were obtained during the revision surgery from the proximal, medial, and distal graft remnants. The samples were evaluated by light microscopy, and the vascularity and maturation of the samples were established by histological scoring.
ResultsThe most common aetiology of reconstruction failure (86.6%) was a specific event with non-contact mechanism. The patients with re-rupture of their ACL plasty less than 12 months after surgery had substance vessels that were less deep. The distal segment of the graft in those patients showed a delay in histological maturation with fewer collagen fibres.
ConclusionIn patients whose ACL grafts failed less than 12 months after surgery, a lower distribution of blood vessels and collagen fibres was found that were less ordered in the distal graft. These results indicate a delay in maturation, which leads to a higher risk of graft failure.
Para la reconstrucción exitosa del ligamento cruzado anterior (LCA) son necesarias la revascularización y la maduración histológica del injerto. Fallos en este proceso pueden causar la rotura del neoligamento.
ObjetivoDescribir las diferencias en la maduración histológica de plastias fallidas precoces (menos de 12 meses poscirugía) y tardías (más de 12 meses poscirugía) en pacientes con rerrotura de reconstrucción de LCA con tendones flexores.
Materiales y métodosEstudio observacional descriptivo. Serie consecutiva de 20 pacientes con fallo en la reconstrucción de LCA con tendones flexores. Muestras obtenidas mediante biopsia de los remanentes del injerto (porción proximal, corporal y distal) durante la cirugía de revisión. Las muestras fueron evaluadas por microscopia de luz y la vascularización y la maduración fueron establecidas mediante un puntaje histológico descrito en la literatura.
ResultadosLa causa más común de fallo de reconstrucción (86,6%) fue un evento identificable sin mediar traumatismo directo. Los pacientes con rotura precoz de la plastia del LCA presentaron vasos sanguíneos más superficiales en comparación con los con rotura tardía. El segmento distal del injerto en los pacientes con roturas precoces mostró una menor maduración histológica con menor número de fibras de colágeno.
ConclusiónEn los pacientes que presentaron fallos en las reconstrucciones de LCA precoces (dentro de los 12 meses poscirugía) encontramos una distribución menor de vasos sanguíneos y fibras de colágeno en la región distal del injerto. Estos resultados indican un retraso en la maduración, pudiendo generar mayor riesgo de fallo del injerto.
The anterior cruciate ligament (ACL) can be reconstructed using different types of graft, although 2 types are mainly used: flexor tendon (ischiotibial) grafts or bone-tendon-patella-bone (BTB)1 grafts. Although the results obtained with these techniques achieve high success rates, there are still questions as to the time required for grafts to mature. This is of fundamental importance in determining when patients can resume their usual activities, as well as because of delays in the process that may give rise to high risks of failure of the surgery and repeat breakage of the LCA.2
Independently of the type of graft used, it must over time gain characteristics similar to those of the native ACL, in a process that is termed ligamentisation.3 The original descriptions of this process were based on animal models with BTB3 reconstruction, and all of the stages for other types of graft have yet to be characterised. Dissimilar results are reported, with times to maturity varying from 6 to 18 months after surgery. Outstanding stages that have been described within the ligamentisation process are ischaemic necrosis, revascularisation, remodelling and maturing, ending in a graft that is histologically similar to the LCA.4,5 The most critical phase is probably graft revascularisation, given that histological and biomechanical maturity depend on this; The original studies described canine models in which graft vascularity originated in the infrapatella fat and rear structures of the joint, while current studies using magnetic resonance imaging (MRI) in reconstructed patients show that flexor graft vascularity stems from branches of the medial and inferior genicular arteries. The first part of the graft to be revascularised is the part within the joint, while those within the bones do so later.6–8 This agrees with histological studies that evaluated ACL arthroplasties, which found that revascularisation within the joint occurs after week 24.9 The authors are not aware of any study that has evaluated the vascularity present in the grafts of failed ACL arthroplasty, and we believe this question to be fundamental to determine the cause of the new breakage. To date this question has not been clarified, and it has been hypothesised to be due to a combination of biological, technical and traumatic factors.
This study aims to determine the histological pattern of graft maturing and revascularisation in 3 different segments of the broken arthroplasty (proximal, medial and distal) in patients with early primary ACL reconstruction failure (during the first 12 months after surgery), and late failures (after the first 12 months following surgery). We hypothesise that in patients with early failure there is a delay in the ligamentisation process.
Materials and methodsThis is a prospective, observational, comparative and blind study (the latter regarding histological evaluation) of a consecutive series of 20 patients with breakage of ACL arthroplasty. Following approval by the Bioethics Committee of our institution, we identified patients with ACL arthroplasty breakage at least 6 months after the primary surgery. This was performed in a single centre by the same surgical team, using autologous flexor tendon graft. A transtibial technique was used, with femoral fixing using Transfix (Transfix, Arthrex, Naples, FL) and tibial fixing with a bio-absorbable interferential screw (Tornillo Biodelta, Arthrex, Naples, FL). All of the patients were evaluated by MRI 6 months after the operation, checking that the graft had been incorporated into the bone and the position of the tunnels. The patients were classified in 2 groups depending on when the arthroplasty broke: early breakages (during the first 12 months after surgery) and late breakages (following the first 12 months after surgery). Their age, sex, mechanism of lesion and the exact time between surgery and breakage of the arthroplasty were recorded.
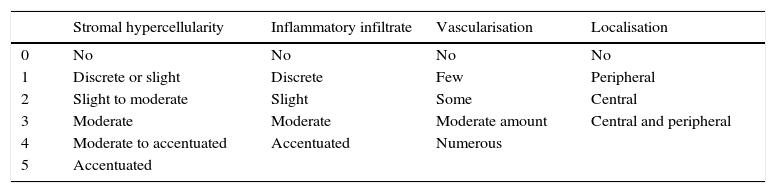
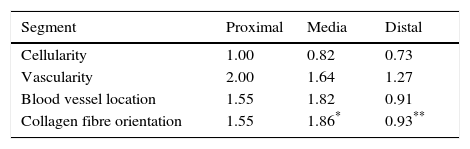
Sample taking and histological evaluationSamples were taken after patients had given their informed consent, during revision surgery and once the remodelled ACL joint had been found to have broken. They were taken for biopsy from the proximal part (close to the femoral tunnel), the body or medial part (in the arthroplasty remnant) and from the distal part (close to the tibial tunnel) from the graft remnant using basket forceps. The samples obtained were fixed in 10% formalin and then analysed by a pathologist who was not aware of the time between primary surgery and breakage or the segment from which the sample came. A series of variables relating to the maturing of the arthroplasty were classified by light microscopy. Vascularity (the number of blood vessels present and their distribution in terms of their depth in the graft) was quantified, together with cellularity (the number of fibroblast-type cells) and the orientation of collagen fibres. Each one of these parameters was classified on a scale of from 0 to 4, where 4 corresponds to tissue with characteristics similar to the ACL according to the descriptions contained in the literature (Table 1).9,10
Points scales assigned to each histological variable.
| Stromal hypercellularity | Inflammatory infiltrate | Vascularisation | Localisation | |
|---|---|---|---|---|
| 0 | No | No | No | No |
| 1 | Discrete or slight | Discrete | Few | Peripheral |
| 2 | Slight to moderate | Slight | Some | Central |
| 3 | Moderate | Moderate | Moderate amount | Central and peripheral |
| 4 | Moderate to accentuated | Accentuated | Numerous | |
| 5 | Accentuated |
Averages were calculated together with their standard deviation. The results were then analysed using the Mann–Whitney U test for continuous samples. The differences with a value of P<.05 were considered to be statistically significant. To determine the correlations between the variables studied we use Pearson's linear correlation, considering a value of r>.80 to be positive for relationships.
ResultsDescription of the patients80% (16 patients) of the 20 patients were men, with an average age of 30.3 years old (19–44 years old). Arthroplasty breakage was due to an identifiable mechanism without direct trauma in 86.6% of cases, and the average time from reconstruction surgery and revision of the ACL was 39.3 months (7–96 months). For those patients who recognised that an event may have corresponded to the moment of arthroplasty breakage, the average time between this event and revision surgery was 3.5 weeks (2–6 weeks).
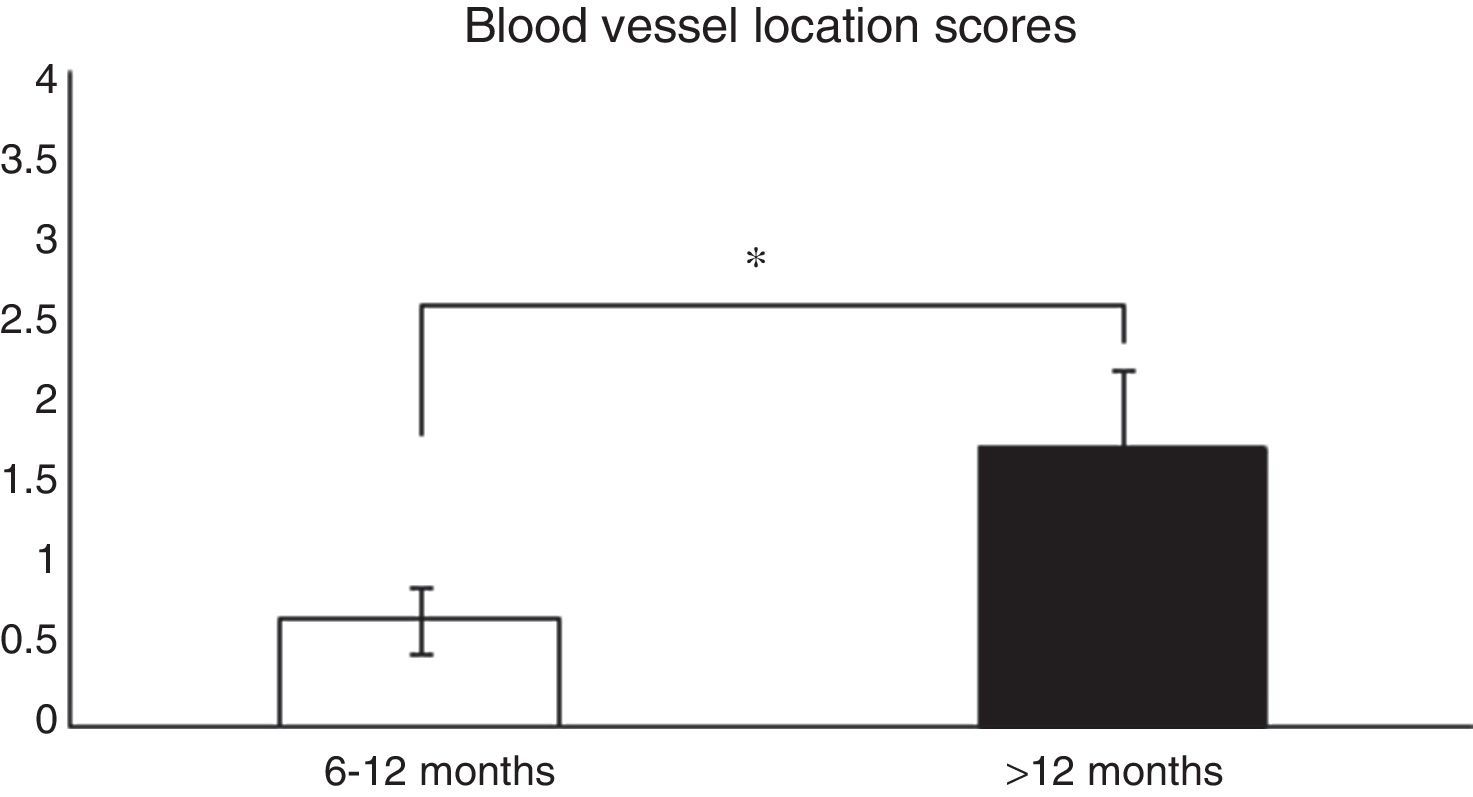
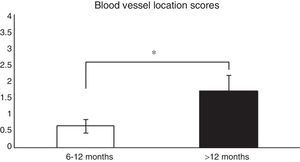
Histological analysis and relationships between the analysed variablesTo analyse whether there were relationships between the time from ACL reconstruction surgery and graft maturity, the samples in general (an average of the 3 segments) were evaluated, and depending on the segment (proximal, medial or distal) from which they had been obtained, before correlating the results with the variables described in Table 1. When the time after surgery was associated with the maturity variables evaluated in the average of the 3 segments, significant differences were found (P=.038) between patients in which the reconstruction had failed before 12 months and those where it occurred after this time in connection with blood vessel location (Fig. 1). When each of the segments was evaluated separately no significant differences were found (P=.071) between them in terms of when the failure occurred.
Comparison of the maturity of ACL evaluated by the location of blood vessels (see the score in Table 1). In patients with graft failure before 12 months we find a significantly lower average score here than is the case for those whose breakage occurred after 12 months (0.66±0.2 vs. 1.7±0.4).
*P=.038.
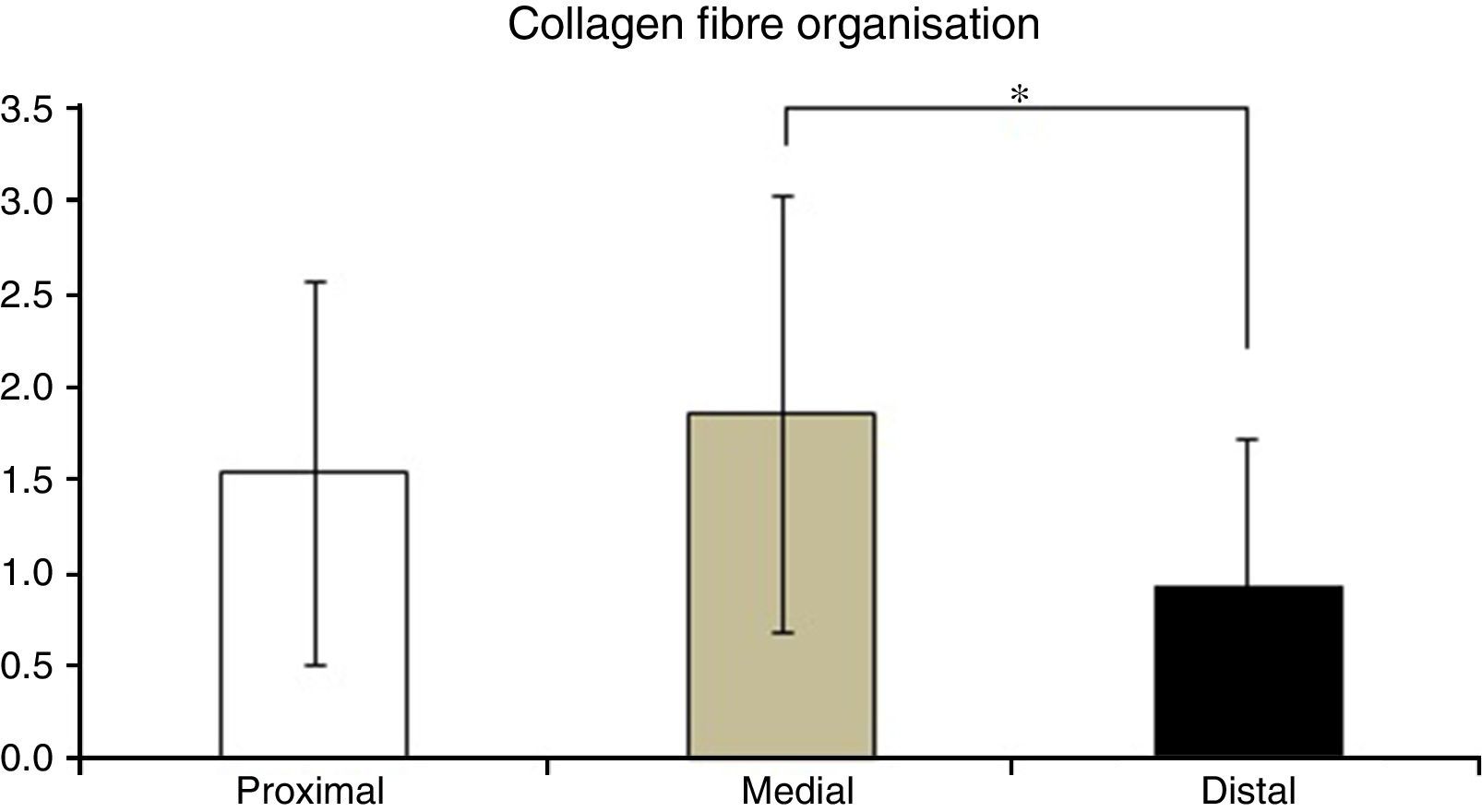
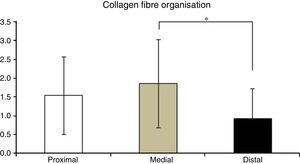
Table 2 shows the averages obtained for vascularity, cellularity and the orientation of collagen fibres in each one of the 3 ACL segments from which samples were taken. When the histological results of each segment were associated with time after surgery no significant differences were found. When the relationship between the variables described in Table 1 and the segment from which samples were obtained was evaluated, significant differences were found in collagen fibre orientation (P=.042), with less maturity in the distal segment in comparison with the medial segment (Fig. 2), although there were no differences between the distal segment and the proximal one, or between the medial and distal one. No differences were found in the other variables analysed.
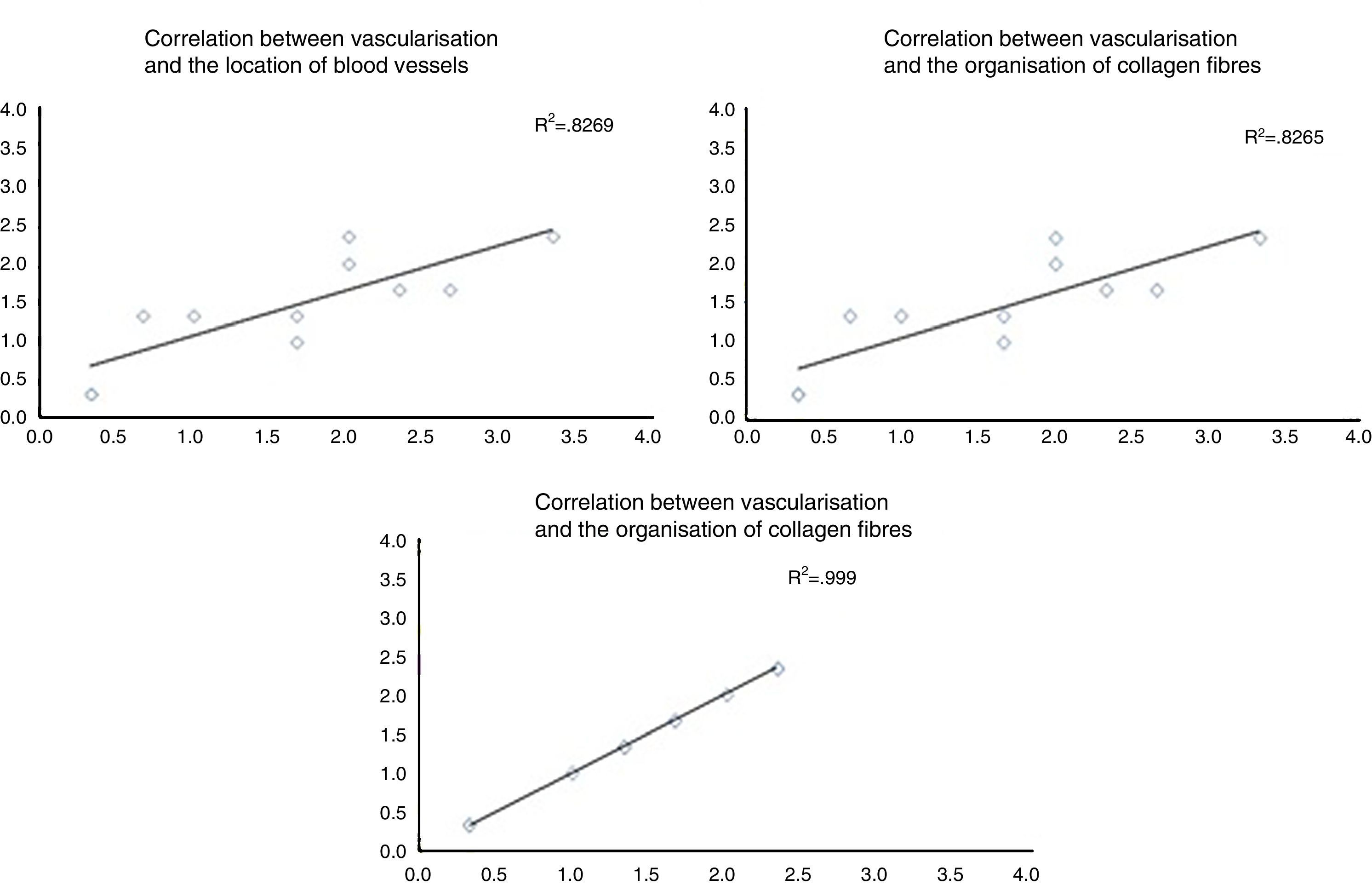
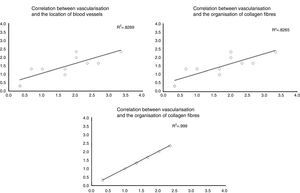
When the correlations between the different histological variables were evaluated, an excellent correlation was found between blood vessel location and collagen fibre orientation (r=0.99), between the amount of blood vessels and collagen fibre distribution (r=0.82) and between the amount of blood vessels and their depth in the graft (r=0.82) (Fig. 3).
The correlation between the different histological variables analysed. When the different histological variables are compared, we find an excellent correlation between the location of blood vessels and the orientation of collagen fibres, the amount of blood vessels and the distribution of collagen fibres, and the amount of blood vessels and their depth within the graft.
Knowing how long grafts used to reconstruct the ACL take to mature seems to be fundamental to reduce the risk of arthroplasty breakage and allowing patients to return to their usual activities more safely. The classical studies in animal models described grafts as suffering a process of necrosis during the first month, continuing with the extra-cellular pattern of tissue, especially the configuration of collagen fibres. During the next 2 months the graft is repopulated by a large number of cells with inflammatory as well as fibroblast characteristics, and the revascularisation process starts. At the sixth month the amount of cells found is similar to that in a native cruciate ligament, even though it still lacks a similar structure. It gains its structure after 9–12 months, when the native tendon is repopulated by synovial cells.11 Nevertheless, to date there has been insufficient evidence of how ligamentisation occurs in clinical practice, or of how any changes in this process may lead to failure of ACL reconstruction surgery. Our aim in this work was to evaluate the relationships between the time after ACL reconstruction surgery and the histological pattern of graft maturing in the 3 different segments of the arthroplasty (proximal, medial and distal) in patients with failure of the primary surgery.
In our sample of 20 patients the main mechanism involved in failure was trauma without direct contact, which is in line with other observations published in the literature.12 Given that all of our patients were evaluated prior to the breakage with a MRI test that showed the proper incorporation of the graft in the bone and the position of the femoral and tibial tunnels, according to the recommendations contained in the literature, we believe that these factors have been ruled out as causes of the ACL reconstruction failure. As several factors play a role in breakage of the arthroplasty, on the basis of our results we believe that failures in our patients were fundamentally due to mechanical and biological causes.13 Of the biological variables that may be involved, we find it interesting that, when maturity is evaluated and more specifically in terms of the location of blood vessels, patients in whom reconstruction failure occurred before 12 months showed less blood vessel penetration towards the graft than did patients in whom failure occurred after 12 months, indicating less graft maturity. Our results differ from the available information in connection with the process of normal ligamentisation in arthroplasty that does not fail; Falconiero et al. analysed 43 ACL graft biopsies and concluded that from 6 to 12 months after the operation there were no significant differences in vascularisation or the orientation of collagen fibres.9
We believe it is possible to suggest that there is a subgroup of patients in whom, for reasons that are not clear, the ACL graft maturing process is delayed. This occurs more in terms of its vascularisation than is the case for its incorporation into the bone, as the latter was achieved at 6 months according to MRI. As the available evidence indicates that the rear part of the graft at the femoral level and the frontal part at the tibial level are the first to revascularise,6 we believe it is possible that failures in this process may increase the risk of graft breakage, which may have occurred in our sample. This may have clinical effects, as it is possible to evaluate graft vascularity using MRI techniques.6–8 Any delays in this process could lead to changes in when patients are allowed to resume doing sport.
Another biological aspect that may be connected with failure of ACL reconstruction in these patients is the difference found in collagen fibre orientation between the distal and medial segments. Biomechanical studies of the flexor tendons used in ACL reconstruction indicate that their collagen content gradually increases as the graft matures, reaching values higher than those of native ACL at from 11 to 13 months after surgery. The lower degree of maturity in the distal segment with fewer collagen fibres, as shown in our study, may lead to a higher risk of arthroplasty failure. This is extremely important in the light of studies which indicate that the distal segment of the graft is the one under the greater biomechanical stresses,5 so that it may be possible that the reconstruction failure observed in our patients is due to delay in maturing at a distal level, as determined by the lower concentration of collagen in this segment.
The methodological weaknesses of our study include the limited number of patients in the sample and the absence of a control group. Nor does it refer to the location in which ACL breakage occurred according to MRI, as studies using this technique have found that the most frequent location of breakage in autologous grafts is the proximal zone. This is similar to what occurs in native ACL, and in allografts a similar number of breakages occur in the proximal and distal zones.14 It may be the case that in our group of patients with autologous grafts the delay in maturity at a distal level may lead to differing imaging behaviour, although this was not determined.
ConclusionsIn our sample of patients with failure of ACL arthroplasty we found a lower distribution of blood vessels in patients whose repeat breakage occurred before 12 months and a lower organisation of collagen fibres at the distal level of the graft. These results may lead to a delay in the maturing process that would lead to higher risks of failure of the surgery. We believe that new studies should be performed to determine the true clinical importance of these phenomena, as well as the possibility of diagnosing them at an early stage.
Level of evidenceLevel of evidence iii.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed are in accordance with the ethical norms of the committee for responsible human experimentation and with the World Medical Association and the Helsinki Declaration.
Data confidentialityThe authors declare that they followed the protocols of their centre of work regarding the publication of patient data.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects referred to in the paper. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Figueroa D, Martínez R, Calvo R, Scheu M, Gallegos M, Vaisman A, et al. Patrón de revascularización de injertos de tendones flexores rotos en reconstrucción de ligamento cruzado anterior: un estudio histológico. Rev Esp Cir Ortop Traumatol. 2016;60:372–377.