INTRODUCTION
Linum usitatissimum (flax) is a plant belonging to the Linaceae family which is used in the textile industry. Hypersensitivity to linseeds, though infrequent, has been known for long1.In our time, linseed oil is increasingly used as a laxative2. Anaphylaxis induced by flax has also been described, and the allergens of both grain3 (in multigrain bread) and oil4 (as a laxative) have been analyzed. In the former report3, weak to mild IgE reactivity to multiple bands in the immunoblotting did not allow for the identification of the involved allergen(s), but it was noted by the authors that the weight of these bands was diminished (from 150-175 to 35-100 kDa) after SH2 reduction of the sample. This reduction in MW after the destruction of disulphide bonds suggested a multimeric protein as the causative antigen.
CASE REPORT
A 39 year-old woman, with a previous history of hay fever and intolerance to egg, suffered an anaphylactic reaction immediately after the ingestion of the first spoonful of grains of linseed, prescribed as a laxative. She had multisystemic involvement (abdominal pain, vomiting, urticaria, dyspnea, dysphonia, dysphagia and hypotension) and fully recovered after being treated at the emergency room.
MATERIALS AND METHODS
Skin prick tests
A battery of commercially available common food allergens (Bial-Aristegui, Bilbao, Spain) was tested with the allergy pricker lancet (Dome-Hollister-Stier) as described5. Prick by Prick tests were also performed with linseed.
Flax extract for in vitro tests
Two grams of linseeds were defatted in acetone and extracted in 20 ml of phosphate buffered saline (PBS) at room temperature (RT). After stirring and spinning, the supernatant was passed through a Millipore filter (0.22 () for sterilization (Millipore, Molsheim, France). The protein concentration, as determined by Bradford assay6 (Bio-Rad, Munchen, Germany) was 3.6 mg/ml.
Total and specific serum IgE
Both were measured by CAP enzymo-immunoassay (Pharmacia Diagnostics AB, Uppsala, Sweden).
Leukocyte histamine release test (LHRT)
LHRT was performed as described7 in order to indirectly detect specific serum IgE antibodies against our linseed extract. Purified horse anti-human IgE (Tago, Burlingame, CA, USA) was used as a positive control. The maximal release was induced by 10 % perchlorate (Sigma, St Louis, MO, USA).
RESULTS
Skin prick test (prick by prick) with linseed was positive, with an immediate response wheal of 5x4 mm in diameter. In addition, the patient had weak positive prick test reactions to three commercial allergens: egg yolk, chicken and mustard. The rest of the skin prick tests performed with other commercial food allergens (animal and vegetal) was negative.
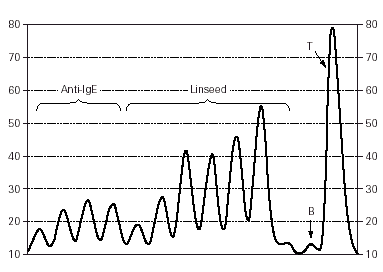
Specific serum IgE against linseed (20 KU/l) and egg yolk (0.46 KU/l), with total serum IgE of 221 KU/l, was demonstrated by CAP assay. A positive Leukocyte Histamine Release Test (fig. 1), with linseed inducing a release of up to 68 % of the total histamine content, further showed the presence of specific IgE.
Figure 1.--Leukocyte Histamine Release Test. On the Y axis, histamine release in the patient's whole blood is expressed in arbitrary units7. The first four peaks show the release after the addition of anti-IgE (positive control)7. The second set of peaks reflects the release of histamine induced by 5-fold growing concentrations of our linseed extract (from 1/15625 to 1/5). The "B" peak stands for the basal release (only whole blood, no stimulus) and the "T" for the maximal release, induced by 10 % perchlorate7.
DISCUSSION
The clinical picture of our patient suggested a type I hypersensitivity, and the demonstration of specific serum anti-linseed IgE corroborated it. An oral challenge, considered unnecessary and risky, was not performed. Our patient did not report any previous contact with linseed, though unaware exposition to this or other possibly cross-reactive allergenic sources must have occurred.
In conclusion, the increasing use of linseed as laxative in herbal medicine (usually without detailed labeling) and in multigrain bread, implies a growing risk of sensitization that should be taken into account.
ACKNOWLEDGEMENTS
F. León is recipient of a grant from the Spanish Fondo de Investigacion Sanitaria (FIS 01/9417).