Undesirable immunological responses to alimentary allergens are one of the hallmarks of atopic diseases. The prevalence of common food allergens is dissimilar among different communities with distinct nutritional habits and genetic characteristics.
AimTo assess the prevalence of the most common food allergens in Iran, using different reliable studies.
MethodsAll studies determining sensitization to common food allergens that were indexed in PubMed, Web of Science, Google Scholar, ProQuest, Scopus, Iran Medex, and Magiran were included in this review. To perform a meta-analysis, STATA 14 and metaprop command was applied. A logistic-normal random-effects model with Freeman–Tukey double arcsin transformation was applied to combine the findings of different studies and evaluate their heterogeneity. Random pooled estimate (ES) (pooled prevalence), 95% confidence interval (95% CI) and p-value were determined.
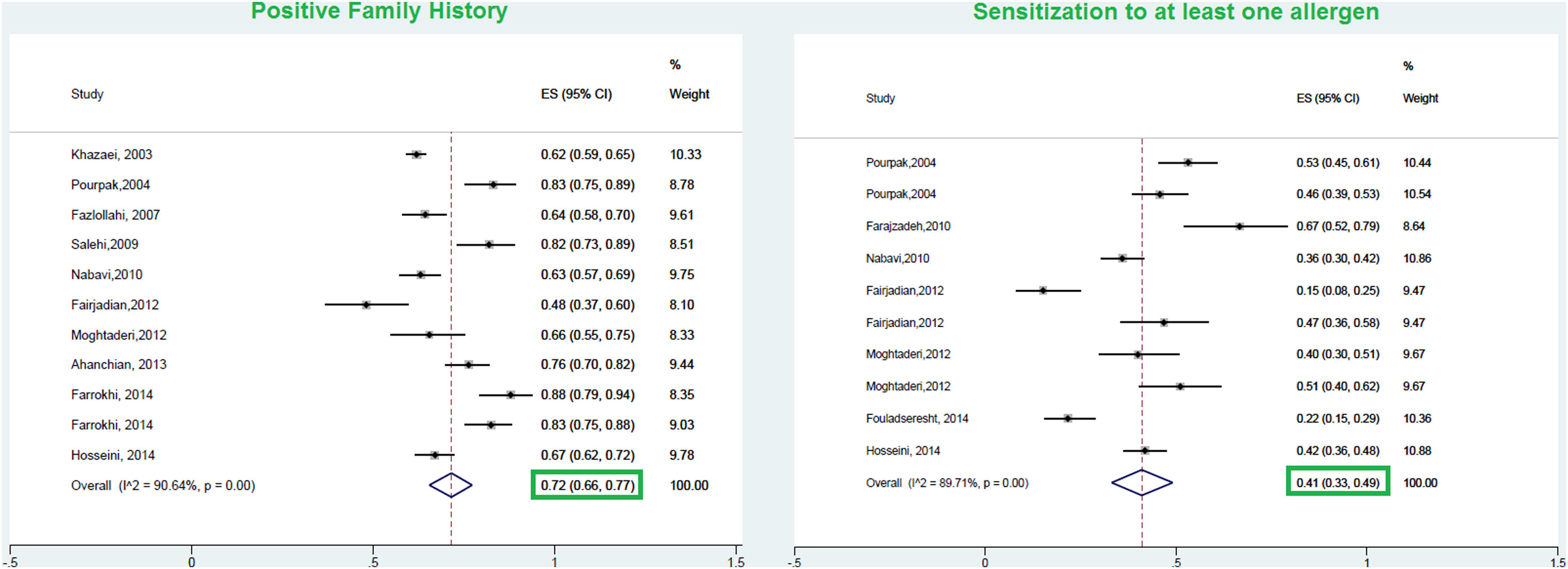
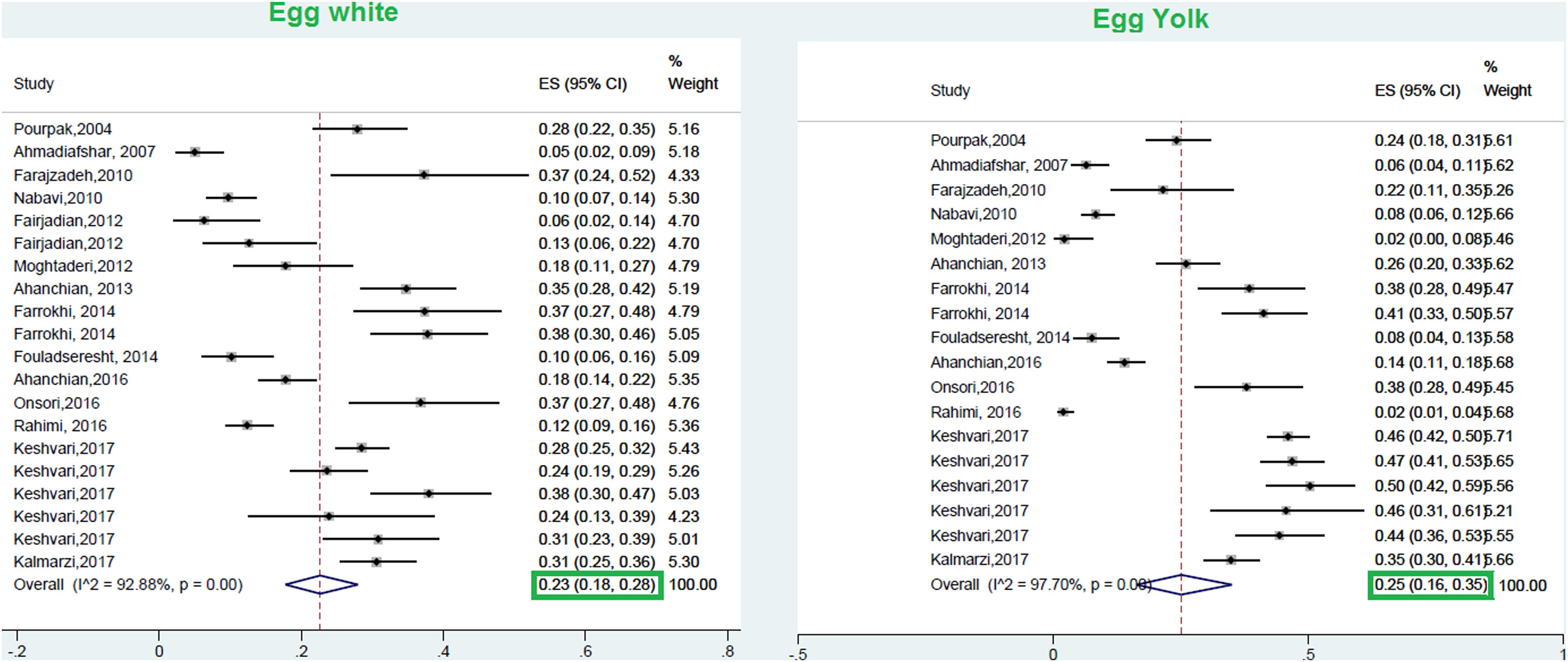
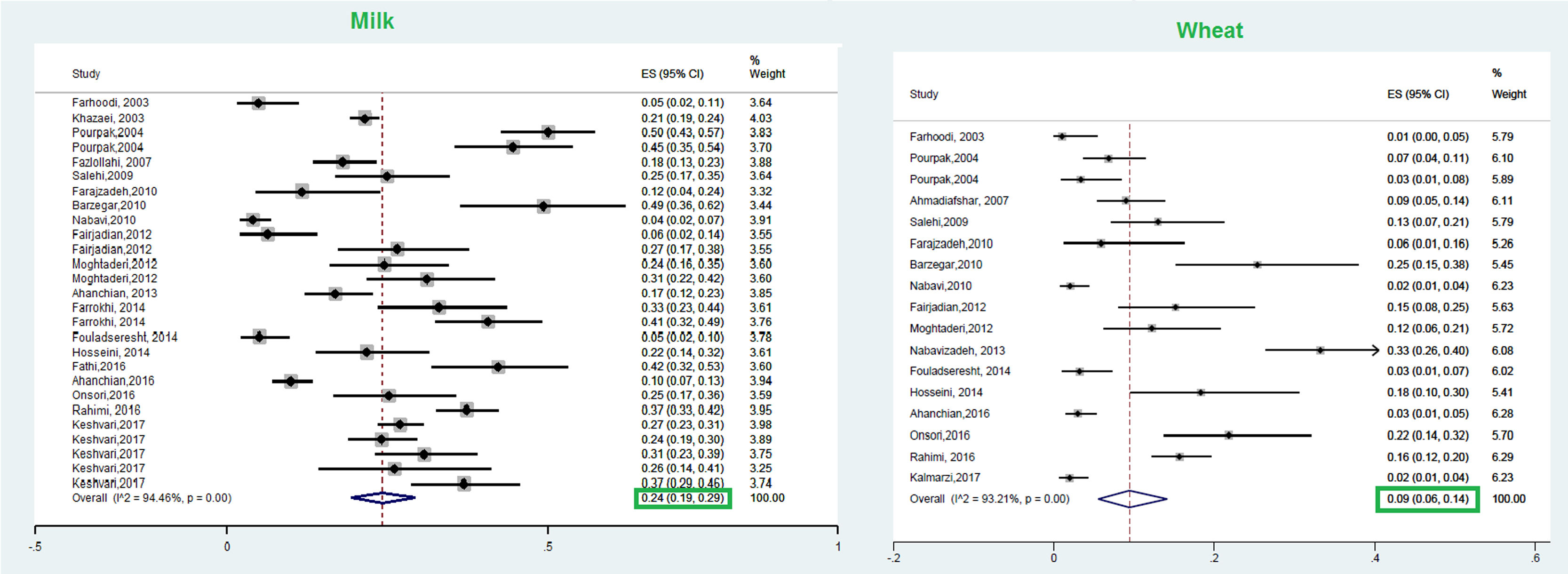
ResultsA total of 23 studies with data from a total of 6126 children and adults met the inclusion criteria for entering this meta-analysis. The respective pooled prevalence of a positive family history of allergy and positive specific IgE to at least one food allergen was 72% (95% CI: 66–77%) and 41% (95% CI: 33–49%), respectively. Our results in the total population revealed that allergic sensitization to egg yolk, cow’s milk (CM), egg white, and wheat were 25% (95% CI: 16%–35%), 24% (95% CI: 19–29%), 23% (95% CI: 18%–28%), and 9% (95% CI: 6%–14%), respectively. Walnut, peanut, and soybean sensitization was detected in 23% (95% CI: 17%–31%), 23% (95% CI: 13%–33%), and 20% (95% CI: 12%–28%) of patients, respectively. Random pooled ES for sensitization to shrimp and fish was 32% (95% CI: 21–45%) and 12% (95% CI: 6–20%), respectively. The result of analysis in different age groups revealed that allergic sensitization to milk, egg white, and egg yolk declines in higher age groups; while shrimp sensitization increases in older patients. In patients with atopic dermatitis, egg white was the most frequent food allergen 29% (95% CI = 18–42%); while wheat was the least frequent 8% (95% CI = 4–14%).
ConclusionsConsidering the prevalence of different food allergens, the results of the current meta-analysis revealed that egg yolk and cow’s milk had the second and third rate after shrimp, respectively. The high prevalence of sensitization to shrimp may be attributed to its high consumption in coastal areas and/or cross-reactivity of shrimp with some aeroallergens such as mites.
Food allergy (FA) as an unexpected immune reaction to foods and increasing health problem affects up to 6–10% of the population in different communities.1 A number of existing studies in the literature have demonstrated a considerable increasing prevalence of FA in recent decades in developed and developing countries.2,3 A cross-sectional study showed a considerably increased prevalence of 3.5%–7.7% for FA in Chinese infants from 1999 to 2009.4 According to the cohort study of Willits et al. in which the temporal trends of FA among citizens of Olmsted County of Minnesota was evaluated, a significantly increased incidence of FA was found between 2002 and 2009 (7%–13.3%), while it became constant afterward.5 It is hypothesized that hygiene status, insufficiency of Vitamin D, and dual-allergen exposure, geographical area, dietary habits, ethnicity, and the rate of antibiotics use could be the reasons for the increasing prevalence of FA.6,7 The highest prevalence of FA has been reported in young children (3–6-year-olds: 26.6%, 7–18-year-olds: 14.7%) rather than adults (11.1%).8 The Isle of Wight Birth Cohort study evaluated the longitudinal trends of FA from birth to the adolescence period; the results of which showed a relatively invariant prevalence of FA in the early years of life (5.3%–5% in 1–4-year-olds) while it showed a significant decrease and increase at 10 years (2.3%) and 18 years of age (4%), respectively.9 According to the study of Sicherer et al., estimating the exact prevalence of FA is difficult as it is affected by a number of factors, including age and ethnicity of the studied population, geographical conditions, applied diagnostic methods, the definition of FA, and common symptoms with other diseases such as gastrointestinal disorders.10
Considering the fact that genetic and environmental risk factors could be the possible triggers of FA11,12 and genetic factors are not amendable, some authors have suggested limiting potential food allergens as an effective strategy to prevent FA.13,14 It has been suggested that different food allergens may induce IgE-mediated hypersensitivity among different age groups.15 According to a recent worldwide report in the general population, cow’s milk (CM) (2.5%), egg (1.3%), peanut (0.8%), soy (0.4%), tree nuts (0.2%), and shellfish (0.1%) are commonly seen among young children; while shellfish (2%), peanut (0.6%), and tree nuts (0.5%) are mostly detected among adult population.16 Additionally, CM and egg were found to be the most frequent allergens in the first 10 years of life; while children aged over 10 years showed a higher prevalence of peanut and tree nuts as well as allergy to shellfish and kiwi appeared during adolescence.9 On the other hand, different food allergens could develop FA in various regions. For instance, Arakali et al. reported a group of unusual food allergens (apple, fish, banana) in South Asia.17
To estimate the prevalence of IgE-mediated FA, several approaches are usually used. Standard questionnaires (including exact clinical history) and specific IgE assay; using in vivo (such as oral food challenge, skin prick test) and in vitro (such as ImmunoCAP and basophil activation test) diagnostic tests are the most important techniques.11,16,18 Double-blind placebo-controlled food challenge (DBPCFC) is determined as the gold standard method to diagnose IgE-mediated food hypersensitivity.19 In addition to the above-mentioned in vivo and in vitro traditional tests, cross-reactivity, sensitization profile to food allergens, and high-risk food allergenic molecules could be determined using molecular tests (such as the microarray) for a diagnosis of molecular allergens.20
In spite of an unproven belief regarding the worldwide increase in the prevalence of FA and its associated public health problems during the last 10–20 years,21,22 the definite prevalence of common food allergens is vague and reliable estimations of their prevalence in Iran are lacking. Considering the fact that alimentary habits are different among distinct countries and even in different parts of a country, determining the most prevalent food allergens is of great importance in the management of allergic patients and can prevent extensive limitations in the regimen; especially in the pediatric group who are at risk of growth retardation. In the present systematic review and meta-analysis, we aimed to determine the most common sensitizations to food allergens in Iranian allergic patients.
MethodsProtocol and registrationWe respected referred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines in this study.23,24 After approving this protocol in the research and ethics committee of Immunology, Asthma and Allergy Research Institute, Tehran University of Medical Sciences approved and registered the protocol of the current systematic review in Tehran University of Medical Sciences (No: 94-04-40- 31266).
Information sources and literature searchA broad scope of the literature search including PubMed, Scopus, MEDLINE, Google Scholar, Iran Medex, and Magiran was applied for reviewing the literature. The search was not limited by language or date of publication and all the manuscripts up to August 2017 were included. Keywords that were used for the database search were as follows: allergen, common, food allergen, Iran, prevalence, specific IgE, and sensitization. Databases were searched by two reviewers and all the full-text articles including original articles, brief communications, and letters to the editor were screened. Additionally, reference lists of relevant articles were also reviewed in order to increase the sensitivity and decrease missing articles. All studies in which the prevalence of food allergens in pediatrics or adults was determined were included. The studies, in which techniques other than SPT, RAST (Radioallergosorbent test), RIDA Allergy Screen, and qLine Allergy (Immunoblotting test), or ImmunoCAP were applied, were excluded from the meta-analysis. These studies were commonly performed on subjects with allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis (AD).
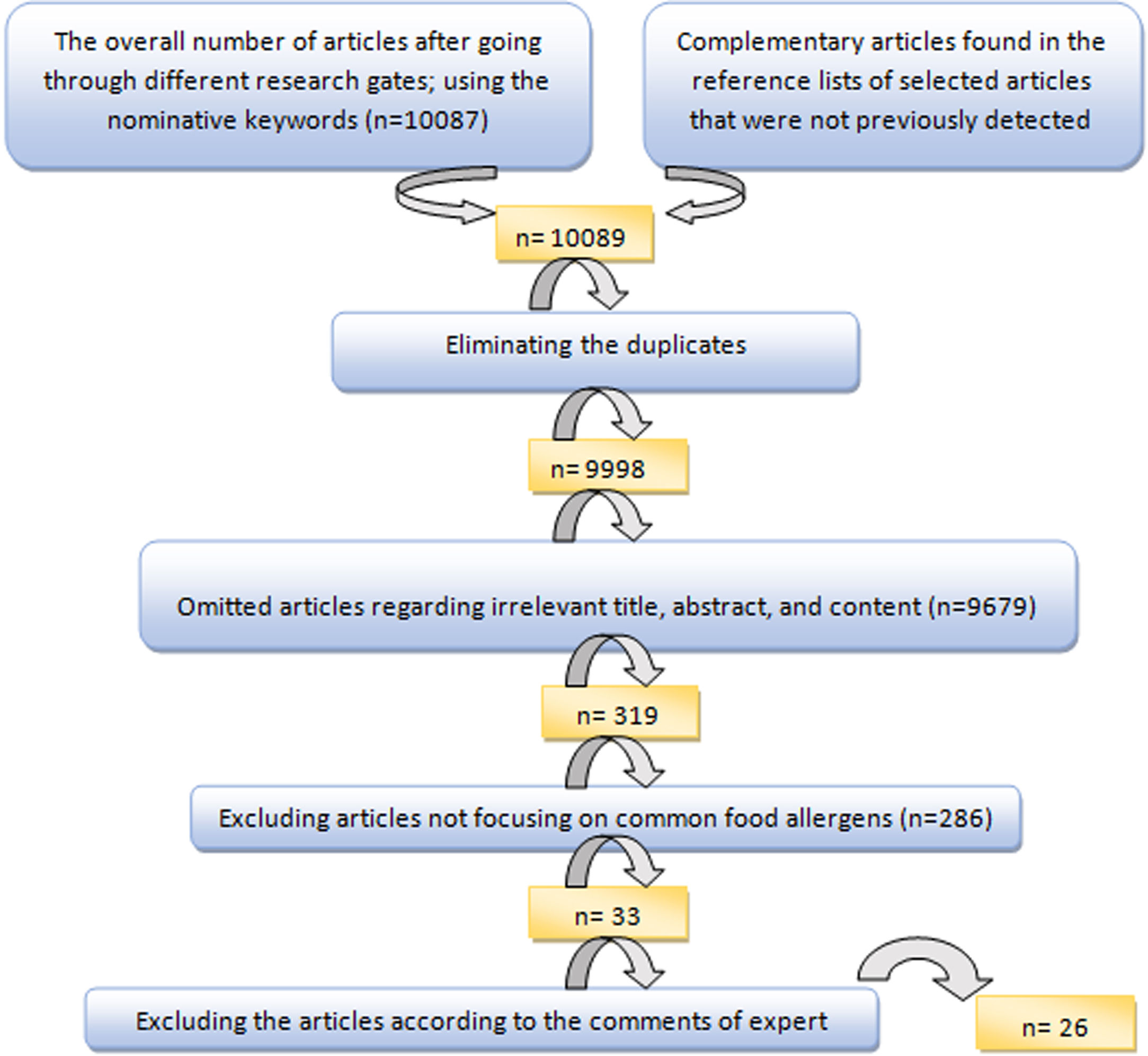
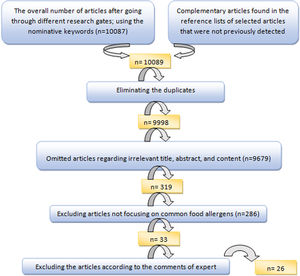
To select the most suitable articles and extract the data of selected articles, an evaluation form, and a data extraction form were applied, respectively. The articles were finally evaluated by the expert committee and the inappropriate articles were excluded after discussing the controversies (Fig. 1).
Data collectionAn Excel data sheet was applied to extract the following data from the finally selected articles: date and region of study, sample size, range of age, sex, applied diagnostic method (in vivo or in vitro), type of allergic diseases, percentage of positive sensitization to at least one allergen, and common allergens (Table 1). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) were applied to determine the quality of the included studies.25
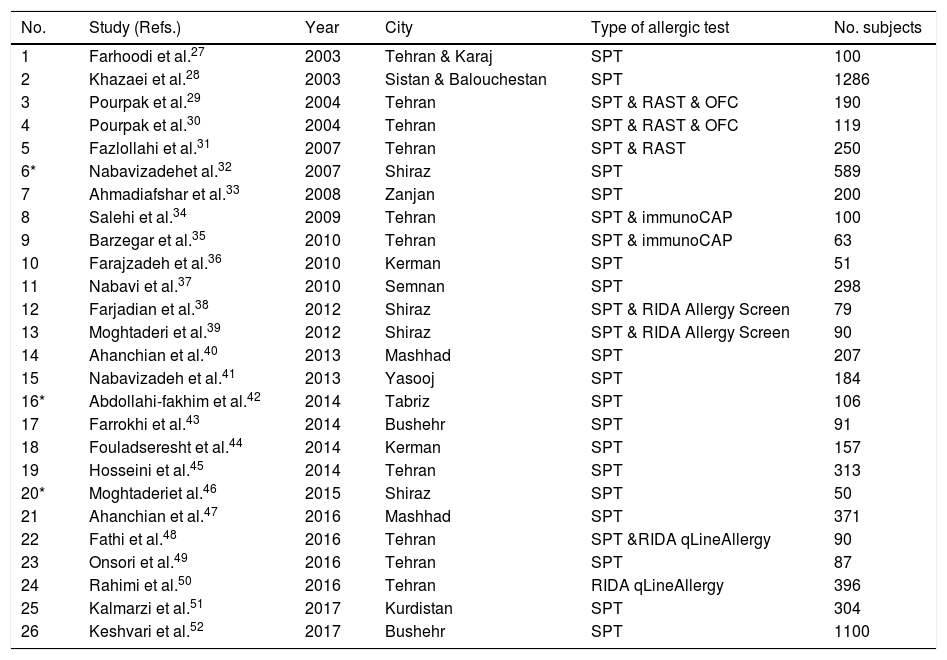
List of included studies in different provinces and cities of Iran and their applied tests.
| No. | Study (Refs.) | Year | City | Type of allergic test | No. subjects |
|---|---|---|---|---|---|
| 1 | Farhoodi et al.27 | 2003 | Tehran & Karaj | SPT | 100 |
| 2 | Khazaei et al.28 | 2003 | Sistan & Balouchestan | SPT | 1286 |
| 3 | Pourpak et al.29 | 2004 | Tehran | SPT & RAST & OFC | 190 |
| 4 | Pourpak et al.30 | 2004 | Tehran | SPT & RAST & OFC | 119 |
| 5 | Fazlollahi et al.31 | 2007 | Tehran | SPT & RAST | 250 |
| 6* | Nabavizadehet al.32 | 2007 | Shiraz | SPT | 589 |
| 7 | Ahmadiafshar et al.33 | 2008 | Zanjan | SPT | 200 |
| 8 | Salehi et al.34 | 2009 | Tehran | SPT & immunoCAP | 100 |
| 9 | Barzegar et al.35 | 2010 | Tehran | SPT & immunoCAP | 63 |
| 10 | Farajzadeh et al.36 | 2010 | Kerman | SPT | 51 |
| 11 | Nabavi et al.37 | 2010 | Semnan | SPT | 298 |
| 12 | Farjadian et al.38 | 2012 | Shiraz | SPT & RIDA Allergy Screen | 79 |
| 13 | Moghtaderi et al.39 | 2012 | Shiraz | SPT & RIDA Allergy Screen | 90 |
| 14 | Ahanchian et al.40 | 2013 | Mashhad | SPT | 207 |
| 15 | Nabavizadeh et al.41 | 2013 | Yasooj | SPT | 184 |
| 16* | Abdollahi-fakhim et al.42 | 2014 | Tabriz | SPT | 106 |
| 17 | Farrokhi et al.43 | 2014 | Bushehr | SPT | 91 |
| 18 | Fouladseresht et al.44 | 2014 | Kerman | SPT | 157 |
| 19 | Hosseini et al.45 | 2014 | Tehran | SPT | 313 |
| 20* | Moghtaderiet al.46 | 2015 | Shiraz | SPT | 50 |
| 21 | Ahanchian et al.47 | 2016 | Mashhad | SPT | 371 |
| 22 | Fathi et al.48 | 2016 | Tehran | SPT &RIDA qLineAllergy | 90 |
| 23 | Onsori et al.49 | 2016 | Tehran | SPT | 87 |
| 24 | Rahimi et al.50 | 2016 | Tehran | RIDA qLineAllergy | 396 |
| 25 | Kalmarzi et al.51 | 2017 | Kurdistan | SPT | 304 |
| 26 | Keshvari et al.52 | 2017 | Bushehr | SPT | 1100 |
SPT: skin prick test; OFC: open food challenge; RAST: Radioallergosorbent test; RIDA Allergy Screen and RIDA qline Allergy (Immunoblotting test).
To perform a meta-analysis of data, STATA 14 (Stata Corp, College Station, TX, USA) and metaprop command were applied. The logistic-normal random-effects model with Freeman–Tukey double arcsine transformation was used to combine the findings of different studies and assess the heterogeneity. Random pooled estimate (ES) (pooled prevalence), 95% confidence interval (95% CI) and p-value were determined. In addition, variable related Forest Plots were drawn. Moreover, the prevalence of food allergens sensitization was analyzed according to pre-school and school-aged/teenagers groups. Additionally, considering the importance of FA in the early years of life, we also analyzed the sensitization to food allergens in 0–3-year-olds. Considering the high prevalence and significant association of AD with FA,26 the allergic sensitization to at least one food allergen, milk, egg white, egg yolk, peanut, and wheat as well as positive family history of FA were separately analyzed in children with AD.
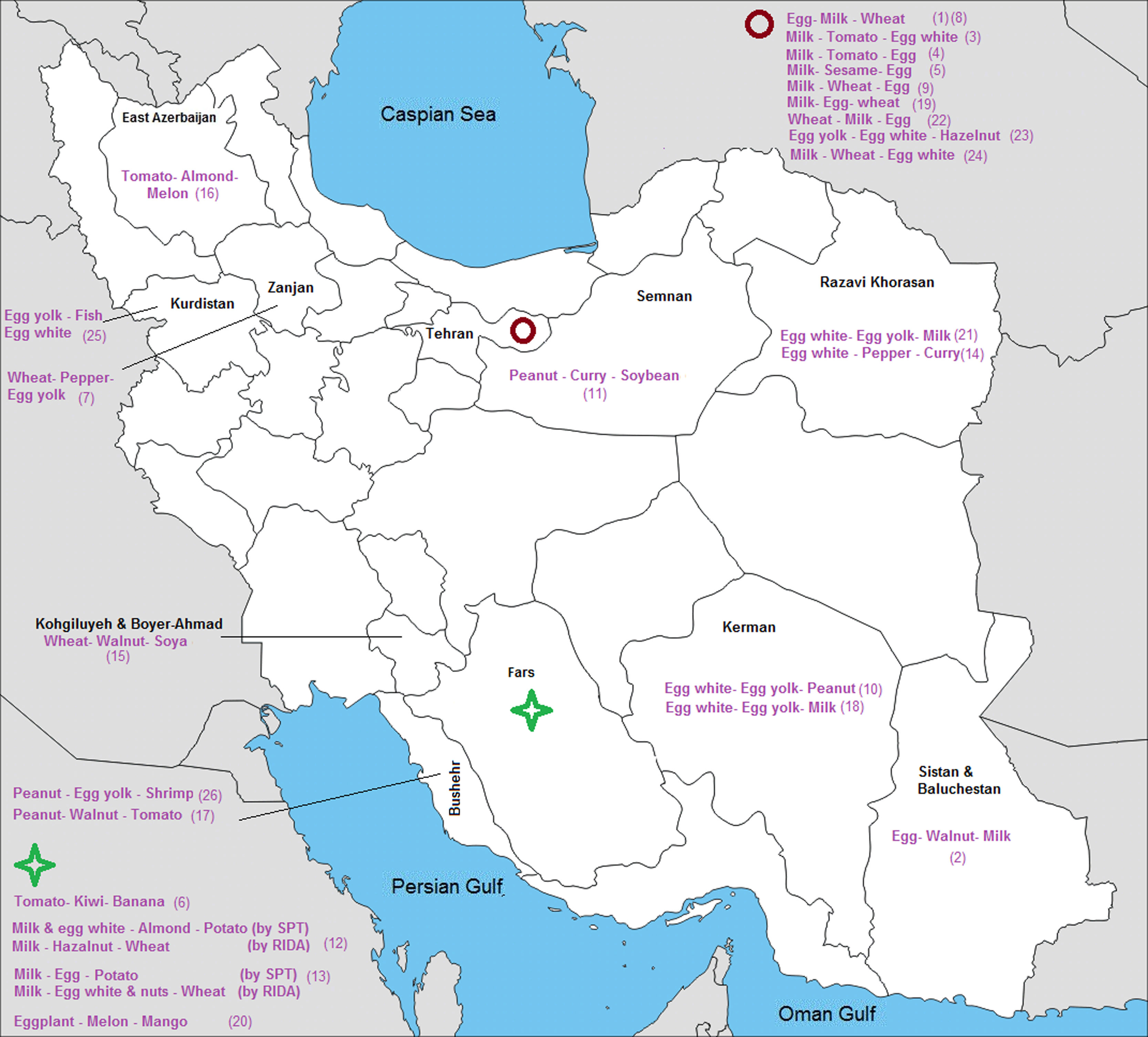
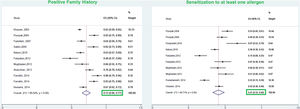
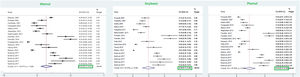
ResultsFrom a total of 10,089 publications in this field, 26 studies were selected and their first three common food allergens were presented in the geographical map, but 23 studies of them with data from a total of 6126 children and adults met the inclusion criteria for entering this meta-analysis. The age of the participants ranged from 1.5 months to 85 years. As Fig. 2 shows, the pooled prevalence for positive family history and positive specific IgE test to at least one food allergen was 72% (95% CI: 66–77%) and 41% (95% CI: 33–49%), respectively; and was statistically significant for both variables (p < 0.001).
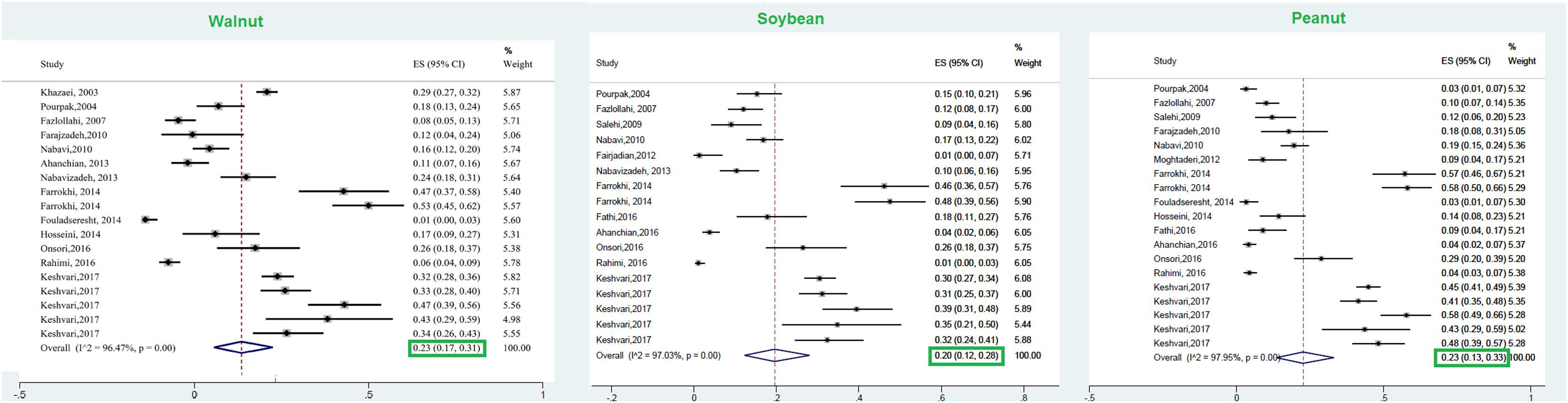
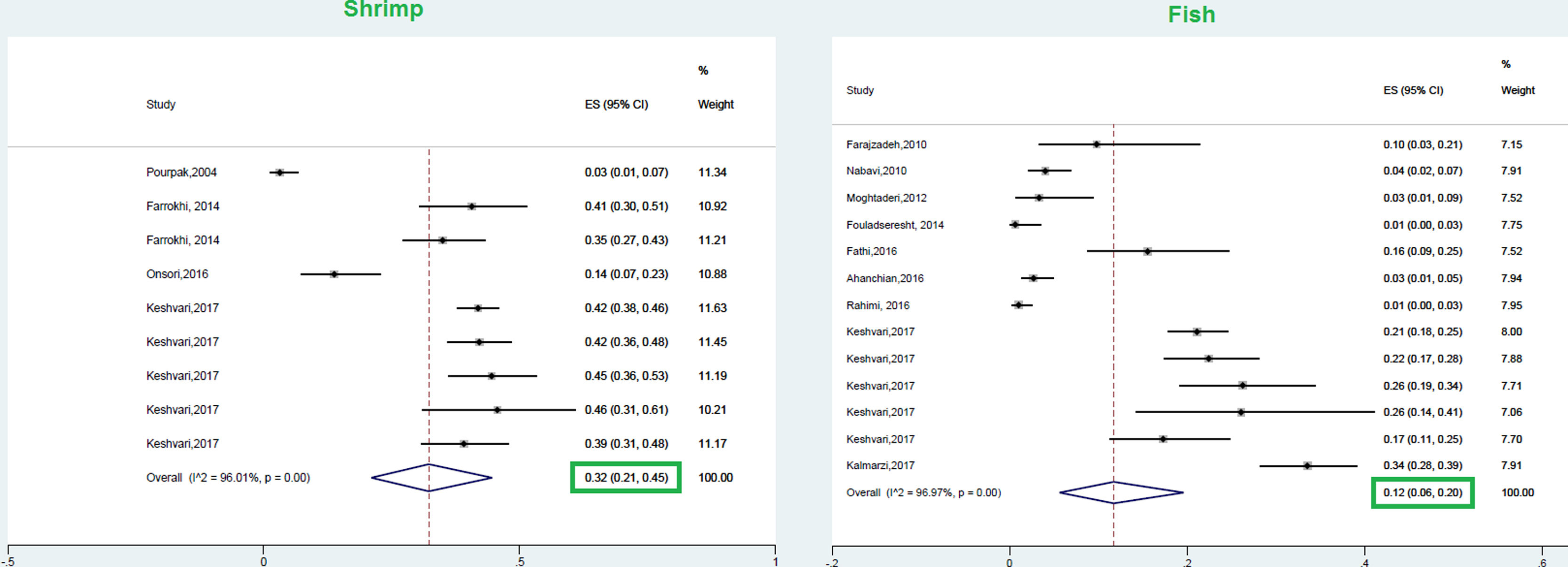
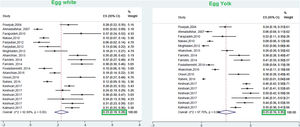
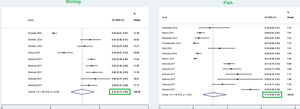
This meta-analysis revealed that the respective random pooled ES in all ages for egg yolk, CM, egg white, and wheat sensitization were 25% (95% CI: 16%–35%), 24% (95% CI: 19–29%), 23% (95% CI: 18%–28%), and 9% (95% CI: 6%–14%), respectively (Figs. 3 and 4). Random pooled ES for allergic sensitization to walnut, peanut, and soybean, were 23% (95% CI: 17%–31%), 23% (95% CI: 13%–33%), and 20% (95% CI: 12%–28%), respectively (Fig. 5). Interestingly, the pooled prevalence of shrimp sensitization was 32% (95% CI: 21%–45%) which was the most common food allergen while the pooled prevalence of fish sensitization as another seafood was 12% (95% CI: 6–20%) (Fig. 6).
As the meta-results shows, the positive family history of allergy and the sensitization to at least one allergen was 77% (95% CI = 70–84%) and 52% (95% CI = 38–66%) in AD children. Moreover, the pooled prevalence of sensitization to egg white, milk, and egg yolk were 29% (95% CI = 18–42%), 25% (95% CI = 17–35%), and 15% (95% CI = 2–36%) in children with AD. Additionally, the allergic sensitization to peanut and wheat were as follows: 12% (95% CI = 8–17%) and 8% (95% CI = 4–14%), respectively.
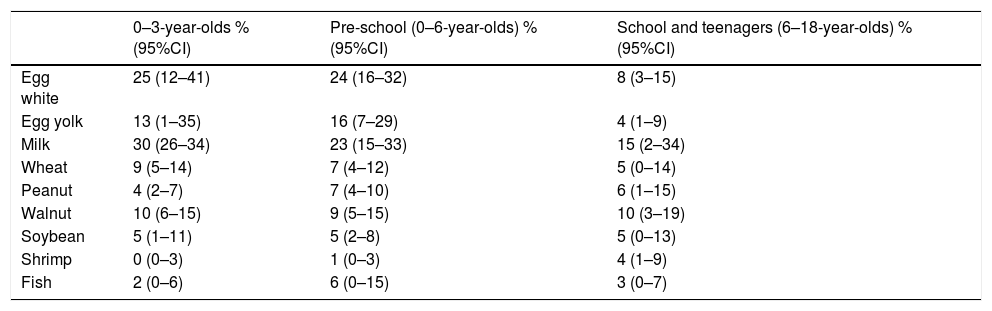
Table 2 shows the results of the meta-analysis for allergic sensitization based on different age groups. As the results show, the allergic sensitization to milk, egg white, and egg yolk decreases with aging; while shrimp sensitization was developed in higher ages.
The prevalence of different food allergens among different age groups.
| 0–3-year-olds % (95%CI) | Pre-school (0–6-year-olds) % (95%CI) | School and teenagers (6–18-year-olds) % (95%CI) | |
|---|---|---|---|
| Egg white | 25 (12–41) | 24 (16–32) | 8 (3–15) |
| Egg yolk | 13 (1–35) | 16 (7–29) | 4 (1–9) |
| Milk | 30 (26–34) | 23 (15–33) | 15 (2–34) |
| Wheat | 9 (5–14) | 7 (4–12) | 5 (0–14) |
| Peanut | 4 (2–7) | 7 (4–10) | 6 (1–15) |
| Walnut | 10 (6–15) | 9 (5–15) | 10 (3–19) |
| Soybean | 5 (1–11) | 5 (2–8) | 5 (0–13) |
| Shrimp | 0 (0–3) | 1 (0–3) | 4 (1–9) |
| Fish | 2 (0–6) | 6 (0–15) | 3 (0–7) |
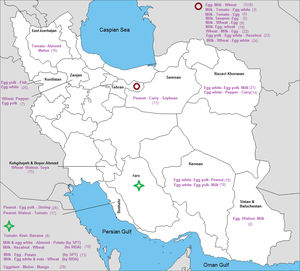
The prevalence of the most common sensitizations to food allergens in different cities of each province is shown in Fig. 7.
DiscussionThe results of the present meta-analysis indicated that positive specific IgE to at least one food allergen was 41% among Iranian allergic patients. In a meta-analysis in Europe, the point prevalence of sensitization to at least one allergen was 10.1% in the general population.53 In a study by Hill et al., the incidence of allergic sensitization to at least one food during the earliest five years of life was reported to be 8.2%.54 According to the findings of a systematic review, IgE sensitization to foods could act as a trigger in developing other allergic diseases such as asthma, atopic dermatitis, and allergic rhinitis.55
The pooled prevalence of positive family history in allergic patients was 72% in the current meta-analysis. There are limited and controversial studies regarding the effect of a positive family history on allergic diseases. According to the study of Koplin et al., children with a positive family history of allergy in one family member showed a moderate odds ratio (OR = 1.4) to develop FA; while a family history of allergic disorders in more than one member was considered as a stronger predictor (OR = 1.8) for developing FA.56 Contrarily, Nissen et al. reported that positive family history was not considered as a strong predictor to develop allergic diseases.57
According to the results of the current study, shrimp had the highest prevalence among food allergens sensitization in all age allergic patients (32%) followed by egg yolk (25%) and milk (24%). In a recent population-based cross-sectional study performed in 1185 children in urban Cape Town and 398 in the urban and rural regions of South Africa, raw egg white (1.9%), cooked egg (0.8%), and peanut (0.8%) were the common food allergens as confirmed by a questionnaire and SPT.58 The results of another recent article revealed that the prevalence of self-reported food allergy among US adults was 19% with shellfish (2.9%), milk (1.9%), and peanut (1.8%) as the most common allergens.59 Similar results were obtained in a meta-analysis performed by Rona et al. the results of which revealed that milk and shellfish had the highest prevalence of food allergy among 51 studies (1.2%–17% and 0–10%, respectively).60 The results of a randomized, cross-sectional study from a total of 38,480 children in US households revealed that peanut, milk, and shellfish allergenicity was higher among children with food allergy (25.2%, 21.1%, and 17.2%, respectively).61
In the next sections, the prevalences of allergic sensitization to the seven most common food allergens are discussed and their respective molecular characteristics are summarized.
Cow’s milk (Bos domesticus)Allergy to CM is considered as one of the most common FA affecting young children.62 The prevalence of this type of FA varies from 1.8%–7.5% in infants aged less than one year old to 0.49%–0.6% in adults.63 Even though this allergy can be detected at any age, most clinical manifestations of CM allergy were initiated during the first year of life.64,65 According to the results of this meta-analysis, the pooled prevalence of CM sensitization was 24% in all ages; while children less than three years showed the highest pooled prevalence (30%). Our previous investigation on patients with CM anaphylaxis revealed that 79.5% had more than one attack; 85.7% of which happened during the first year of life.66 In a study by Yong et al.,8 lower prevalence (4.9%) of CM sensitization was found in allergic patients aged 7–18 years old, which was found to be 15% in the current study. Along with wheat flour, CM has been known as the main elicitor of anaphylaxis in Iranian children.35 In the study of Lee et al., CM allergy was introduced as the second food allergen among Asian children.62 In a large study by Sun et al., a 4.8% prevalence of cow milk sensitization was obtained in subjects with suspected allergic reactions, this was less than in our study.67 On the other hand, in a systematic review in Europe, the lifetime prevalence of self-reported CM allergy and point prevalence of positive SPT to CM was 6.0% (95% CI: 5.7–6.4) and 0.33 (95%CI: 0.03, 0.64) in the general population, respectively.68 One of the most important reasons for this difference could be attributed to the evaluation of sensitization or allergy in population-based and patient-based studies.
According to the literature review, more than 90% children with AD showed food allergic reactions to one of the allergens including cow’s milk, hen’s egg, peanut, wheat, soy, nuts, and fish.69 The reported prevalence of atopic dermatitis in children ranged between 6 and 19% in different cities of Iran.70–72 As the results of this study demonstrated, the prevalence of milk sensitization was 25% (95% CI: 17–35%) in AD children. In a study by Mavroudi et al., 39.28% of food-sensitized patients with AD showed CM allergy.73
More than 40 types of proteins have been recognized in CM, the most important of which include caseins (Bos d 10), α-lactalbumin (Bos d 4), β-lactoglobulin (Bos d 5), bovine serum albumin (BSA) (Bos d 6), lactoferrin (Bos d Lactoferin), and immunoglobulins (Bos d 7)74 (www.allergome.org). According to the results of our recent study performed in 87 children with CM allergy, α-lactalbumin and casein were the most frequent allergenic molecules, while there was a significant association between specific IgE positivity to β-lactoglobulin and anaphylactic reactions.75 Considering the fact that casein is the most common molecular allergen in CM, anaphylactic reaction to β-lactoglobulin may seem strange. However, recent studies have shown that 37% of β-lactoglobulin molecules remained unbroken under conventional heating.76
Hen's egg (Gallus domesticus)Hen’s egg proteins are considered to be one of the most important triggers of FA in pediatrics.77 The prevalence of egg allergy has been estimated at about 1.3%–10% in different studies.78 The results of this study revealed that the pooled prevalence of sensitization to egg white and egg yolk in allergic patients was 23% and 25%, respectively, while a 1.38% prevalence of egg sensitization was reported in suspected patients to allergic diseases.67 The results of a systematic review in Europe performed in the general population revealed that the lifetime prevalence of self-reported egg allergy and the point prevalence of positive SPT to egg were 2.5% (95% CI: 2.3–2.7) and 0.76 (0.63, 0.88), respectively.68 Interestingly, a study in Australia has reported a high prevalence of 9% in their population.79 According to the study of Lee et al., egg was reported as a predominant food allergen in most regions of Asia including China, Korea, and some southeast Asian countries.62 According to a systematic review in Asia, 23% of asthmatic patients of Hong Kong and 49.2% of Taiwanese patients with atopic eczema showed allergic sensitization to egg and milk.80 The results of this study considered egg white as the most common food allergen (29%) in children with AD.
The prevalence of allergic sensitization to egg white is considered as the second common food allergen in patients 3–6 years (23%) and 7–18 years (9.8%),8 which was similar to our meta-analysis results in these age groups (24%, 8%).
Interestingly, the prevalence of egg white and egg yolk sensitization was higher in the studies of coastal cities, which was compatible with Yong et al. study.8
Twenty-three glycoproteins have been identified from egg white.81 Gal d 1 (Ovomucoid), Gal d 2 (Ovalbumin), Gal d 3 (Ovotransferrin), and Gal d 4 (lysozyme) are the most important allergenic molecules of egg white.81,82 Chicken serum albumin (Alpha livetin, Gal d 5) is considered as the main egg yolk allergen.83
Wheat (Triticum aestivum)Wheat allergy is considered as an important problem due to the wide usage of this cereal worldwide especially in Asia, the early entrance of wheat in the human diet, and the occurrence of severe allergic reactions as a result of a concomitant allergy to other kinds of food allergens.84,85 In spite of the fact that wheat allergy is highly prevalent during childhood some reports have also mentioned rare cases of IgE-mediated reactions in adults.86 The results of the present meta-analysis revealed that the pooled prevalence of wheat sensitization was 7% and 9% in the pre-school allergic children and all age allergic patients, respectively. Similarly, the prevalence of wheat sensitization was found to be 7.9% in 3–6 years allergic children in Taiwan.8 The self-reported wheat allergy and positive IgE results obtained from a population-based meta-analysis in Europe 3.6% (95% CI: 3.0–4.2) and 3.91% (3.38- 4.44).68 On the other hand, a variable prevalence of wheat allergy has been reported in different Asian countries. Among them, Japan reported wheat as the dominant allergen in school children; while Taiwan, Hong Kong, and China showed a low prevalence of wheat allergy.62 Pourpak et al. introduced rice and corn as the best alternatives to wheat in wheat-allergic patients; while similar reactions to barley were observed in patients with wheat allergy 87.
A total of 28 different allergens have been introduced up to now (www.allergen.org). Tri a 19/Omega-5gliadin (65 kDa) is the major wheat allergen being recognized in patients with a wheat allergy and wheat-dependent exercise-induced anaphylaxis.88 Tri a 14 (9 kDa) was found as a lipid transfer protein (LTP) and major allergens individuals with a wheat allergy and Bakers’ asthma.88 In addition, alpha-amylase inhibitors (chloroform methanol soluble) are known as major components causing this syndrome.89
Walnut (Juglans nigra/Juglans regia)This tree nut belongs to the Juglandaceae family, including 24 species all of which are edible.90 Juglans regia (Persian or English walnut) and Juglans nigra (the black walnut) are two walnuts that are mostly cultured in temperate climates.90 According to a global systematic review, allergy to walnut was the most prevalent of nuts in the USA (37–48%) and UK (24%).91 According to multicenter research in Europe (EuroPrevall), the prevalence of walnut allergy was 2.2% in young adults of the general population.92 Accordingly, the highest prevalences of walnut allergy were detected in France (3.7%), Germany (3.3%), Italy (3.1%), and Spain (3.1%).92 On the other hand, tree nuts were uncommon allergens in most East Asian countries such as Korea, China, Singapore, and Taiwan.62 However, the results of the current meta-analysis revealed that the prevalence of walnut sensitization in allergic patients was 23%, which was lower than that of the USA. This difference could be due to the studied population (general population vs. allergic population) in our study and other studies.
Juglans regia has several allergic molecules, the most important of which are listed below: Jug r 1 (2S albumin) and Jug r 2 (Vicilin seed storage protein) are considered as major allergenic molecules of walnut (www.allergen.org). According to the study of Pastorello et al., which was conducted in a geographical area with a high rate of sensitization to Lipide transfer protein, IgE binding to Jug r 3 (lipid transfer protein) was detected in 91.3% of patients with walnut allergy.93 Jug r 4–6 are other molecules with less allergenicity (www.allergen.org).
Peanut (Arachis hypogaea)Peanut is classified as a member of the Fabaceae family which has anti-inflammatory, anti-cancer, and antioxidant properties.94 In spite of the useful properties of peanut, it stands for almost a quarter of all food allergens with a possibly increasing rate according to current research.61,95,96 Despite the high prevalence of peanut allergy in western countries, its frequency is far less in Asia.62 According to a cross-sectional study among 3218 children with FA in the United States, 24.8% of individuals had peanut allergy, with the highest frequency reported in the 6–10-year-old children96; while the prevalence of peanut sensitization was 6% in 7–18 years-old-children. Burney et al. reported that the overall prevalence of IgE sensitization to peanut in the young adult population of Europe, USA, and Australia was 2.6%.92 In the present meta-analysis, the total prevalence of allergic sensitization to peanut was 23% in allergic patients.
To date, 17 distinct peanut molecular allergens have been recognized (www.allergen.org/www.Allergome.org). Ara h1, Ara h2, and Ara h3 as members of storage proteins family are considered as major allergens in patients with peanut allergy. In addition, these proteins are highly believed to cause life-threatening reactions.97 Ara h 5 (Profilin), Ara h 6 (2 s albumin), and Ara h 7 (2 s albumin) have even less allergenicity in susceptible patients (www.allergen.org). Ara h 8 is a Bet v 1 family member with allergenicity in 85% of patients.98 Peanut-allergic patients from the Mediterranean area are mostly sensitized to Ara h 9 as one of the major peanut allergens.99,100 According to the study of Kukkonen et al., simultaneous sensitization to Ara h 2 and Ara h 6 could predict severe allergic reactions to peanut.101
Soybean (Glycine max)Soybean is considered as one of the eight foods that are responsible for the most important allergic reactions to foods in the US and Europe.102 Despite the numerous advantages of Legume consumption in providing a healthy dietary habit, they can induce life-threatening allergic reactions as well.102 The prevalence of allergy to soy was 0.4% (95% CI: 0.3–0.6) as stated in a population-based meta-analysis of European countries.68 The results of this study revealed that the pooled prevalence of sensitization to soybean in allergic individuals was 20%. The pooled prevalence of peanut sensitization in the pre-school allergic children of this study (5%) was much lower than in the Yong study (26.6%, 14.7%).8
The eight allergenic molecules (Gly m1–8) of soybean have been recognized and registered in IUIS Allergen Nomenclature and Allergome websites.102 Gly m 1 (hydrophobic protein, 7 kDa) (www.allergen.org) and Gly m 4 (PR-10 protein, Bet v-1 like protein,17 kDa) are considered as the major allergenic molecules of soybean in patients with soybean allergy.103 Ito et al. suggested that there is a relationship between specific IgE to Gly m 5 and 6 (7S and 11S globulins) and severe clinical symptoms in Japanese children and may be applied as excellent prediction testing for cases suspicious to soybean allergy.104 Gly m 7 (seed biotinylated protein, 76.2 kDa) and Gly m 8 are considered as major allergens in patients with soybean allergy.105
FishAs a precious nutritional source of proteins, amino acids, fatty acids, and vitamins, fish plays a main role in the human diet; especially in coastal areas.106,107 The self-reported lifetime prevalence and of fish allergy SPT positivity to fish were 2.2% (95% CI: 1.8–2.5) and 0.6% (95% CI 0.5–0.8) in a population-based study in Europe, respectively.68 However, our results showed that this statistic was 12% in Iranian atopic patients. In spite of the high consumption of fish in Asian Countries (especially East Asia), the prevalence of fish allergy is not common.62 The population-based prevalence of fish allergy in three countries including the Philippines, Singapore, and Thailand were 2.29%, 0.26%, and 0.29%, respectively.108 According to a review on fish allergy, the highest population-based prevalence (self-reported) of fish allergy was in Norway (3%) and Philippine (2.29%); while the highest prevalence of IgE sensitization has been reported in Africa.109 On the other hand, the prevalence of fish allergy was 5.6% in Australian allergic children.109
Parvalbumin is considered as the main allergen in different species of fishes.110 Different allergenic molecules with parvalbumin structure (such as Gad m1, Sal s 1) were recognized in a variety of fishes.110 In addition to parvalbumin, tropomyosin has been considered as an allergenic molecule in some fishes (such as tilapia).110 However, approximately 70% of patients showed IgE binding to collagen, tropomyosin, aldolase A, or β-enolase for cod and tuna.111
ShrimpAllergic reaction to shrimp is considered as a kind of shellfish allergy with a prevalence of 1.3–5.2% in different countries. The allergic reactions to shellfish allergens are usually serious and life-threatening.112 According to this study, in the study of Chokshi et al., the occurrence of shrimp anaphylaxis was meaningfully higher in adults with shrimp allergy compared to the pediatric population.113 According to the results of the present meta-analysis, shrimp had the highest pooled prevalence of food sensitization among Iranian atopic individuals, at 32%. Interestingly, shrimp and peach were found to be the second common food allergens after hazelnut in the population-based study of Europe, USA, and Australia with a prevalence of 5.4%, which was different in Western Europe, USA, and Australia.92 Shellfish is considered as a very important allergen in Asia and Asia Pacific countries.114,115 In a study in Taiwan, the prevalence of IgE sensitization to shrimp was found to be 62.7% in pediatrics with atopic dermatitis.116 Shrimp sensitization was considered as one of the most common food allergens in 3–6-year-old allergic children in the Yong study, which is much higher than this study (1%). Considering the fact that shrimp is not commonly found in the food basket of Iranian families, the high prevalence of shrimp allergy in this systematic review may be attributed to the high consumption of this aliment in coastal areas. Bearng in mind the high prevalence of mite sensitization in Iranian atopic individuals [38% (95% CI: 21%–57%)]; especially in coastal areas 129, this high prevalence may be attributed to the cross-reaction between shrimp and some allergens such as house dust mites.116 Accordingly, a concomitant unexpected allergy to fish and/or shrimp is seen in patients with poultry meat allergy that may be caused by the high similarity of low molecular weight proteins such as myosin light chains.117 The majority of studies evaluating shrimp sensitization have been carried out in coastal cities where the prevalence of mites is high. Considering the cross-reaction between mites and shrimp, the high prevalence of shrimp in these areas seems reasonable.
Regarding a variety of shrimp species, different allergenic molecules have been recognized and named. Tropomyosin has been considered as the most famous allergenic molecule of different shellfishes (such as Pen a 1, Pen m 1). In addition to shellfish, this pan-allergen has been recognized in mites and cockroaches.115,118 In addition to tropomyosin, several allergenic molecules such as Arginine kinase and Myosin light chain have been found in crustaceans.119
Cross-reaction and cross-sensitizationAn exact allergic reaction to related allergens in allergic patients to particular foods (such as shrimp with some crustaceans, shrimp with house dust mites, etc.) is defined as a cross-reaction; while a positive specific IgE (using in vivo or in vitro tests) in patients who can tolerate related foods without any symptoms is representative of cross-sensitization.20,120 Cross-reactivity or cross-sensitization could be developed as the result of the presence of cross-reactive molecules such as profolin, PR-10 proteins, Lipid Transfer Proteins, tropomyosin and etc.20 Bet v 1 family and profilins that are not resistant to heat denaturation and gastric enzymes are the main allergens involved in cross-reactions between pollens and food allergens.121 Additionally, sequence homology of Art v 3 as the main allergen of mugwort pollen with lipid transfer proteins from apple, plum, and peach has caused a high cross-reactivity between these allergens.122 Cross-reactivity is considered an important matter that should be taken into consideration for favorable food avoidance without unnecessary limitations in patients’ diet.120
Limitations and conclusionOne of the important limitations of the current study is that the prevalence of food sensitization was evaluated in the allergic group and the prevalence of common allergens was not determined in the general population in any of the included studies; as performed in other studies around the world. OFC studies were limited in Iran and only two studies were found in our literature review. In addition, there were few studies on FA in patients with AD.
According to the results of the current meta-analysis, shrimp was the most common food allergen among Iranian allergic patients. However, considering the fact that shrimp is not a common food in alimentary habits of an Iranian family, this high prevalence may be attributed to its high consumption in coastal areas and/or cross-reaction of molecular allergens of shrimp with other aeroallergens, particularly noteworthy is the mites group.
Conflicts of interestThe authors have no conflict of interest to declare.
FundingThis study was supported by a grant from Tehran University of Medical Sciences (No: 94-04-40- 31266). None of the authors has direct or indirect commercial financial incentive associating with publishing the article, and no funding agreement limits our ability to complete and publish the study.
Ethical disclosuresThe protocol of this systematic review was approved by the research and ethics committee of Immunology, Asthma, and Allergy Research Institute and approved and registered by Tehran University of Medical Sciences (No: 94-04-40-31266).
Right to privacy and informed consent. Not applicable.
We highly appreciate Dr. Kimia Gohari for her kind collaboration in this work.