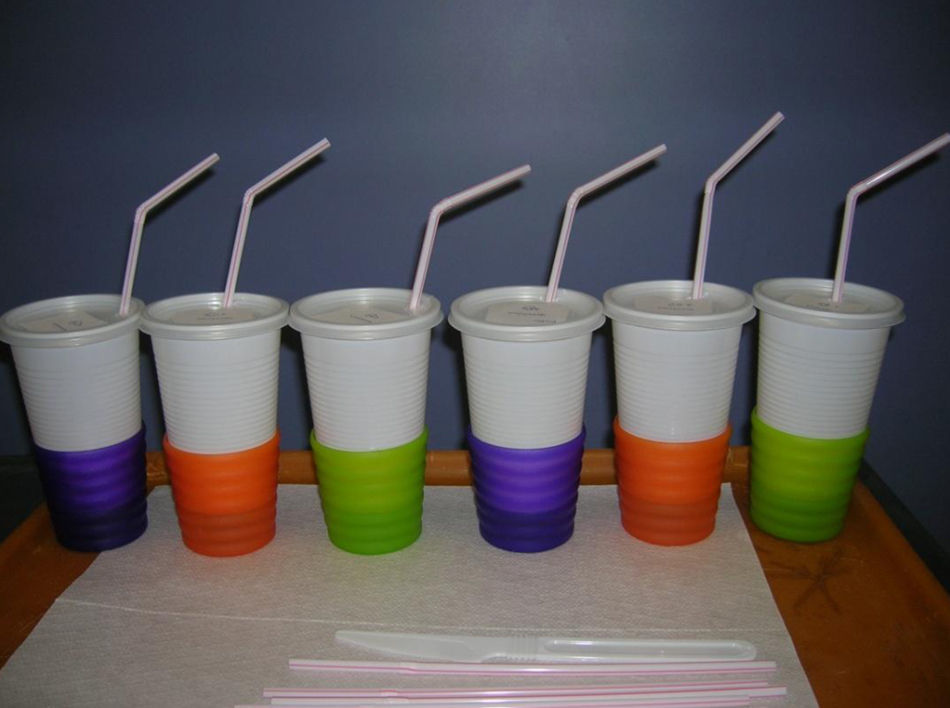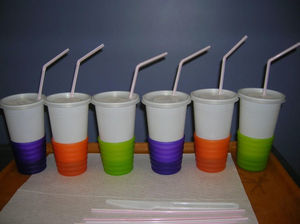A double-blind, placebo-controlled food challenge (DBPCFC) is considered the gold standard for diagnosing food allergy, but because of methodological difficulties it is rarely conducted in clinical practice, especially in paediatric patients. The purpose of the study was to propose a DBPCFC protocol that is adapted to our conditions for the diagnosis of an IgE-mediated cow's milk allergy (CMA) in a Brazilian reference centre for paediatric allergies.
MethodsThis study includes the experimental phase (choice of materials, adjustments made to protocols described in the literature) and the test execution phase. DBPCFCs were performed in 58 patients aged 1–15years who were separated into two groups: Group 1 (n=39), sex 1.6 M:F, 5.3years median age, suggestive history of IgE-mediated CMA; and Group 2 (n=19), sex 1.4 M:F, 8.3years median age with symptoms not associated with milk ingestion and laboratory data not compatible with IgE-mediated CMA.
ResultsThe materials were standardised for testing: containers and disposable products, low-lactose cow's milk (CM) and vehicles, such as natural fruit juice, vegetable soup and soybean-based beverages. Each DBPCFC was performed in a single day with two blind, randomised phases with a 2-h interval between them. The milk doses were gradually increased and offered in regular intervals of 15–30min. Following negative or inconclusive results, patients underwent an open oral challenge test with 200mL of low-lactose CM.
ConclusionsThe proposed adaptation for the DBPCFC allowed to implement this important test for the diagnosis of IgE-mediated CMA in a reference centre for paediatric allergies. It was considered feasible and safe if performed in an appropriate setting with physician supervision.
Cow's milk allergy (CMA) is the most common food allergy among children, with a prevalence of approximately 3% in the first year of life.1 Several immunological mechanisms may be involved in CMA, but mediation by immunoglobulin E (IgE) is the most common.2 A diagnosis of CMA is based on epidemiological, clinical and laboratory findings. Although a double-blind, placebo-controlled food challenge (DBPCFC) is considered to be the gold-standard method for CMA diagnosis, there are still many unanswered questions and many difficulties that accompany oral food challenge procedures.3,4 The main objective of a DBPCFC is to reproduce symptoms that are triggered during natural exposure without any interference from the patient, family or the physician responsible for the examination. Its implementation consists of two parts: the food to be investigated and a placebo, which are both added to a vehicle in increasing doses and at regular intervals. The vehicle must mask the characteristics of the food or placebo and allow the tested food to be offered in small volumes, in a quantity that is sufficient to cause symptoms. The test sequence is known only by the person responsible for randomisation. The high cost, the time consumed, and the lack of standards are the main limitations of DBPCFC. Because potentially severe reactions may occur, it is essential to provide a setting with adequate infrastructure and trained staff that are able to respond to anaphylactic reaction. In children, one of the biggest challenges is choosing the vehicle used to conceal the food, because capsules are not viable. In many studies, the test food is added to other foods.3–5 However, increasing the dose of the food being tested is difficult because its characteristics become harder to mask.
Although DBPCFCs are an important tool for the diagnosis of CMA patients and follow-up, they are seldom performed in clinical practice because of the methodological difficulties; in particular, special adjustments are required for paediatric patients.
Thus, the objective of this study was to propose a protocol for implementing a DBPCFC for the diagnosis of IgE-mediated CMA in a Brazilian public reference centre for paediatric allergies.
MethodsIn a period of six years, 60 children and adolescents between the ages of 1 and 15years were recruited to participate in this study. Thirty-nine were in attendance at the clinic of a Food Allergy (FA) referral centre for paediatric allergy. Nineteen healthy children were also selected to compose the control group.
The patients included were divided into two groups: Group 1: 39 patients with history suggestive of IgE-mediated CMA and tested positive for IgE specific to cow's milk (CM) proteins (CM for prick test and/or fractions ≥3mm of the negative control and/or ImmunoCAP® ≥3.5kU/L to CM and/or fractions). The following characteristics suggested a history of IgE-mediated CMA: anaphylaxis triggered by CM or personal atopy and/or family history associated with clinical manifestations triggered up to 2h after ingestion of the CM. Anaphylaxis was defined according to the criteria described by Sampson et al.6 Group 2: 19 patients with clinical history data not suggestive of CMA (symptoms not consistent with IgE-mediated CMA, including non-specific symptoms after ingestion of CM, no appearance of reactions with derivatives of CM or complaints after 6h of ingestion).
The exclusion criteria were based on those proposed by Niggemann and Beyer4 including recent anaphylactic reaction (in the last 2years) triggered by any agent associated with concentrations of total or specific IgE in food or inhalants or uncontrolled asthma.
Two patients refused the portions offered during the test and were excluded.
The median age was 5.3years in Group 1 and 8.3years in Group 2. The gender distribution was similar between both groups (M:F of 1.6 and 1.4 in Group 1 and Group 2, respectively). Atopy family history was positive in 72% of patients in Group 1 and 63.2% of patients in Group 2. Personal atopy affected about 77% and 47.4% of patients from Groups 1 and 2, respectively. The median age of CMA onset was 4months in Group 1.
The materials, a suitable setting for the test and the schedule for placebo and formula test administration were chosen in the experimental phase of this study. Potential containers and vehicles were discussed by the researchers, and tasting sessions of the placebo and CM preparations by the staff. The decision of which vehicle to use was based on fulfilment of the criteria that define a good vehicle.5 The opinion of parents about the preference of children was considered.
The tests were performed at the day hospital, which fulfilled the necessary conditions for treating serious reactions and were comfortable for patients and families. Additionally, there are many activities to entertain the children while they are tested, such as drawing materials, computers and television. An adequately equipped hospital kitchen was located next to the day hospital. All medical and nursing professionals remained with patients during the test. We could count on the medical staff of the intensive care unit located near the site of examination. A nutritionist was responsible for the preparations used in the tests, and her presence was occasionally requested during the examination for the preparation of a replacement vehicle. All legal caregivers for children under 18years of age remained with the patients for the entire examination.
To avoid cross contamination during the test preparation, milk was manipulated and stored at different times, using disposables. The required test portions were prepared on the test day and stored in a refrigerator. The necessary equipment and materials for the portion preparation included opaque white cups (300mL) with a lid, opaque straws, all disposable. Coloured and opaque cup holders were used. Both the odour and the appearance of the test portions, especially the colour, were masked in these materials (Fig. 1). When the patient was a younger child, a bottle covered by opaque paper was used. The DBPCFC also used syringes, a refrigerator, a microwave oven, disposable tags for identification, liquid and food vehicles to mask the CM. As vehicle options, we used vegetable soup, pudding, soy extracts juices Tonyu-Yakult® and fruit juice Del Valle®. They are found in supermarkets in 200mL Tetrapak™ packaging. CM with low lactose used was Zymil-Parmalat®.
The equipment and medication necessary for treating severe reactions were available during the tests, including pulse oximetry, intubation equipment, a defibrillator, and epinephrine (1:1000) for intramuscular use. Venous access was maintained during the test period for all patients.
A checklist was used to evaluate patient conditions just before the test and is described in Table 1, based on Williams and Bock.7 The use of maintenance medications for chronic diseases was maintained at a dose that would not compromise interpretations of the test. If any condition was not fulfilled, the test was cancelled and rescheduled.
Checklist for the DBPCFC.
| 1. The food to be tested was strictly avoided during the previous 2weeks? |
| 2. Anti-histamines were avoided in the past 10days? |
| 3. The patient is in good healtha? |
| 4. The consent form was signed? |
| 5. Companion over eighteen years old? |
| 6. Equipment and drugs for the treatment of severe reactions available? |
| 7. Patient fasted for at least 6h? |
| 8. Venous access obtained? |
A clinical evaluation was performed through a detailed interview before testing. FA data were obtained, such as the volume or quantity of trigger food and the time interval between ingestion and the onset of symptoms, clinical manifestations, time interval since the last reaction, and drugs used. A physical examination was performed for all patients before testing, including evaluation of vital signs.
The following laboratory tests were obtained before the DBPCFC: blood eosinophil count using Leishman staining, total IgE serum level by ELISA, specific IgE to CM, α-lactalbumin, β-lactoglobulin and casein (ImmunoCAP® and skin prick test). The prick test was conducted according to Pepys8 using IPI-ASAC (International Pharmaceutical Immunology, SA) extracts. The ImmunoCAP® (Phadia Diagnostics, Uppsala, Sweden) was performed as described in Axen et al.9 The positivity criterion was based on that of Sampson and Albergo,10 which considers positive values ≥3.5kU/L.
Execution of the DBPCFC test for cow's milkThe drafting of the DBPCFC protocol was based on the literature data, primarily on the methodology described by Williams and Bock7 (Table 2).
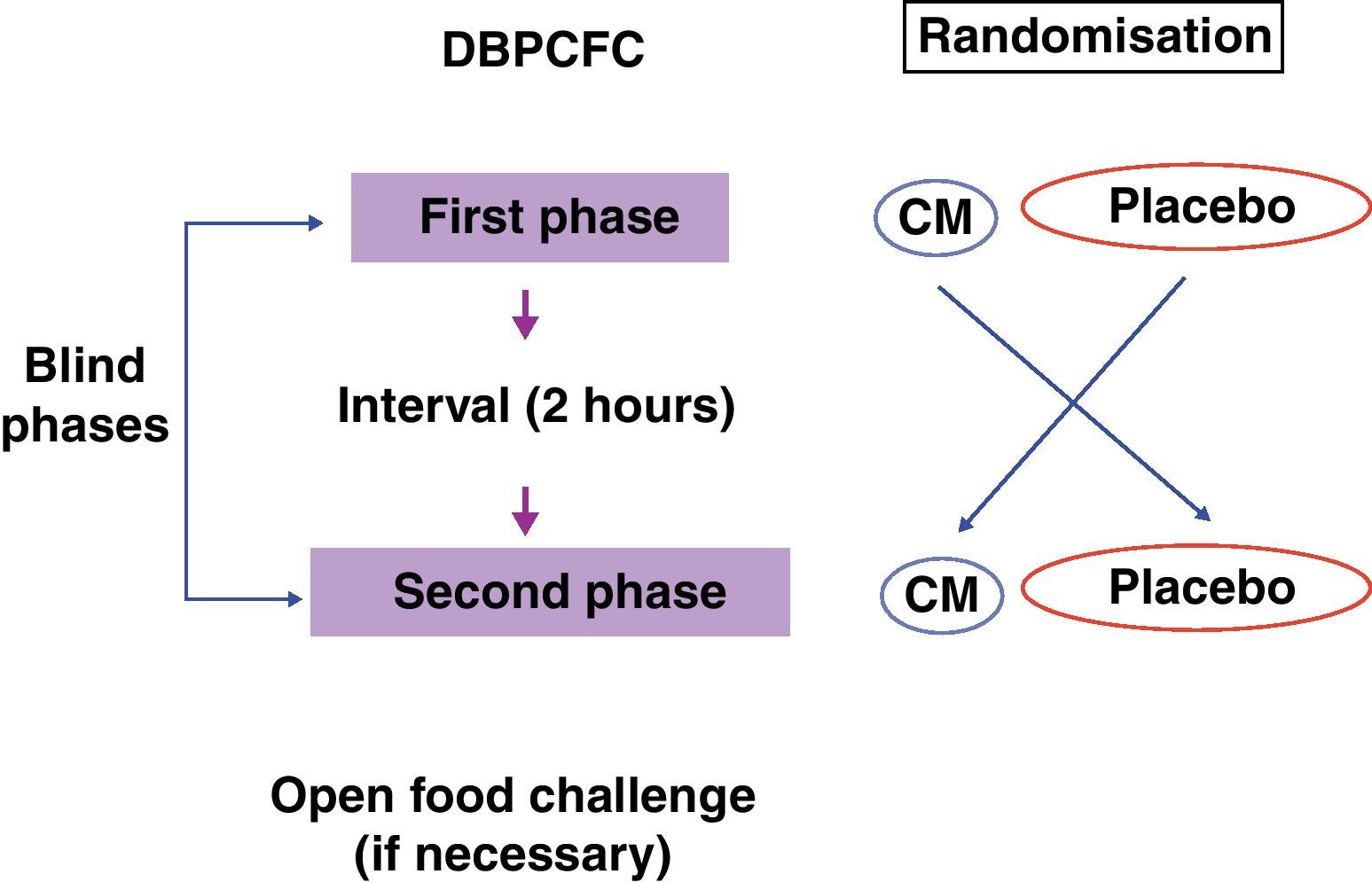
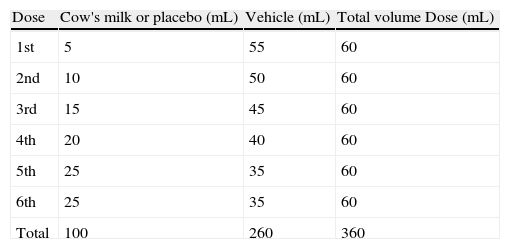
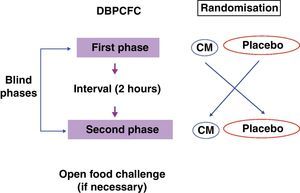
The recommended steps consist of two phases, a blind phase and an open phase that are defined as follows: CM blind phase: Six doses with increasing volumes of CM were added to the vehicles (total=100mL CM, equivalent to 10g of lyophilised CM), and a placebo blind phase included six doses with increasing volumes of water added to the vehicles. Open phase: One dose with 200mL CM (equivalent to 20g of lyophilised CM) was offered (Fig. 2).
The nutritionist was responsible for preparing and randomising the sequence of blind phases. The volume of each dose is shown in Table 2.
The test portions were identified on the basis of the randomisation. The physician, the nursing staff and the patient were unaware of the portion contents offered with the vehicle. Only after the end of both phases was the sequence revealed. The test began in the morning and lasted 90–180min for each blind phase with a 2-h interval between phases. During this interval, patients received a light meal of hypoallergenic rice, cooked chicken and vegetables (broccoli, carrots, zucchini or chayote), lemon juice and lemon gelatine without additives or fruit known to trigger clinical symptoms. An identical meal was offered to parents and caregivers to prevent patients from ingesting unknown food items.
Patients who did not experience a significant reaction during the blind phase then underwent the open oral food challenge, receiving 200mL of CM. After the test, the patient remained under observation for 2h before they were discharged from the hospital. The total test duration, including the two blind phases and the open phase, was approximately 8h.
Tests were considered positive in patients whose objective symptoms reproduced, in part or in full, those in the patient's clinical history, starting up to 2h after the CM ingestion. Objective symptoms were those observed by the medical staff, such as urticaria, angio-oedema, bronchospasm, stridor, runny nose, sneezing, nasal obstruction, conjunctival injection associated to lacrimation, persistent vomiting and diarrhoea. The onset of any objective symptoms justified the interruption of the test, and the patient was treated if necessary. Subjective or unobservable symptoms included pruritus without apparent skin lesions, abdominal pain and nausea.11 The presence of subjective symptoms or isolated perioral papules did not justify test interruption.7
At discharge, the patient was instructed to maintain a restricted diet until the return visit 1week later, and during this interval every symptoms were to be reported to a physician by telephone. On the return visit, the test result was reported to the patient and family, and in cases with positive results, the exclusion diet was maintained with a calcium replacement if necessary. In cases with negative results, the diet was released.
This study received the approval of the Ethical Committee from the Hospital das Clínicas, School of Medicine, Universidade de São Paulo, and the patients’ guardians signed an informed consent form after being informed in a comprehensible manner about the study, including the possibility of severe reactions during the DBPCFC and the availability of emergency care by a qualified medical team.
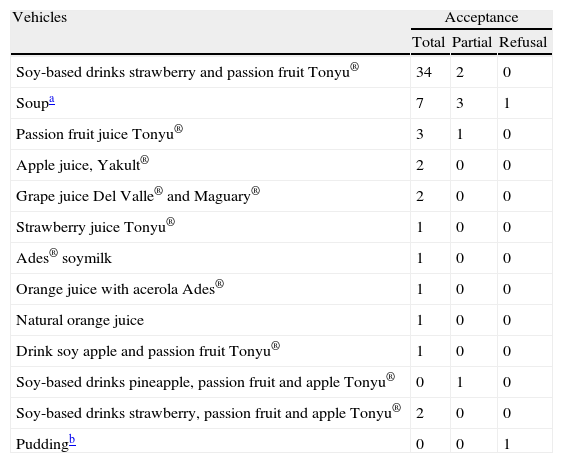
ResultsAmong the vehicles tested, those using soy extract juice and the soup (chicken, turkey, carrots, chard, rice, parsley, oil and salt) were better accepted by patients (Table 3). Additionally, natural fruits juices were also offered as an option and were well-accepted. Soy was an option only for patients whose soy allergy diagnosis had been excluded. In this study, coloured and opaque cup holders and cup lids ensured that the preparations were masked. With these materials, both the portion odour and the appearance, especially the colour, were masked. When the patient was a younger child, a bottle covered by opaque paper was used. In this study, we initially chose to use full CM skim reconstituted with water. Chocolate or yogurt was used to finalise the test (open provocation). During the study, five patients were observed to have gastrointestinal symptoms (colic and diarrhoea) during the CM phase, and these symptoms were not mentioned in the clinical history that was previously obtained. We hypothesised that these patients were lactose intolerant, and a CM with low lactose levels was adopted to eliminate this possible confounding factor.
Acceptance of vehicles used by patients.
| Vehicles | Acceptance | ||
| Total | Partial | Refusal | |
| Soy-based drinks strawberry and passion fruit Tonyu® | 34 | 2 | 0 |
| Soupa | 7 | 3 | 1 |
| Passion fruit juice Tonyu® | 3 | 1 | 0 |
| Apple juice, Yakult® | 2 | 0 | 0 |
| Grape juice Del Valle® and Maguary® | 2 | 0 | 0 |
| Strawberry juice Tonyu® | 1 | 0 | 0 |
| Ades® soymilk | 1 | 0 | 0 |
| Orange juice with acerola Ades® | 1 | 0 | 0 |
| Natural orange juice | 1 | 0 | 0 |
| Drink soy apple and passion fruit Tonyu® | 1 | 0 | 0 |
| Soy-based drinks pineapple, passion fruit and apple Tonyu® | 0 | 1 | 0 |
| Soy-based drinks strawberry, passion fruit and apple Tonyu® | 2 | 0 | 0 |
| Puddingb | 0 | 0 | 1 |
Some patients were discharged before the start of the test for the following reasons: not signing the informed consent form by the patients’ guardians, use of antihistamine, contact with CM with recent reaction or presence of signs and symptoms that could compromise the interpretation of the test such as fever, diarrhoea, respiratory symptoms, exacerbation of co-morbidities such as asthma and rhinitis. In these situations, tests were then rescheduled for another day.
The DBPCFC followed the protocol described by Williams and Bock7 with some adjustment: the time interval between doses from 10 to 15 or 30min, and the volume of CM and placebo doses from 5, 10, 20, 20, 20, 25mL to 1 (for patients with previous history of anaphylaxis), 5, 10, 15, 20, 25, 25mL.
In cases in which the patient had difficulty in accepting the offered portion, the amount accepted was noted and the next dose given. The main difficulty was in the open phase when 200mL of CM low-lactose was offered. Often due to aversion, it was necessary to offer a second option that was a strawberry yogurt (Itambé®) or chocolate Toddynho®.
All children in Group 1 exhibited clinical reactivity in the CM phase, but none of the children in Group 2 exhibited reactivity in CM phase of the DBPCFC.
For assistance in interpreting and carrying out the tests, it was necessary to classify the events in objective (anaphylaxis, angio-oedema, urticaria, cough, stridor, recurrent vomiting, wheezing, dyspnoea, diarrhoea, hives, watery eyes, red eye, runny nose, sneezing, persistent obstruction) and subjective (nausea, sensation of itching without skin lesions, abdominal pain, itching of the oropharynx). Reaction severity was graded as follows and was based on Sicherer et al.12: severe, moderate, mild, and minimal. The reactions that occurred 2h or more after immediate symptoms were considered as delayed reactions. In this study, five patients (13%) had these reactions, in a time interval ranging from 2h15min to 24h and with the following manifestations: itching, angio-oedema, cough, wheezing, sneezing, runny nose, diarrhoea, vomiting. Among the 44 patients who performed the placebo phase, five had reactions at this stage (Table 4).
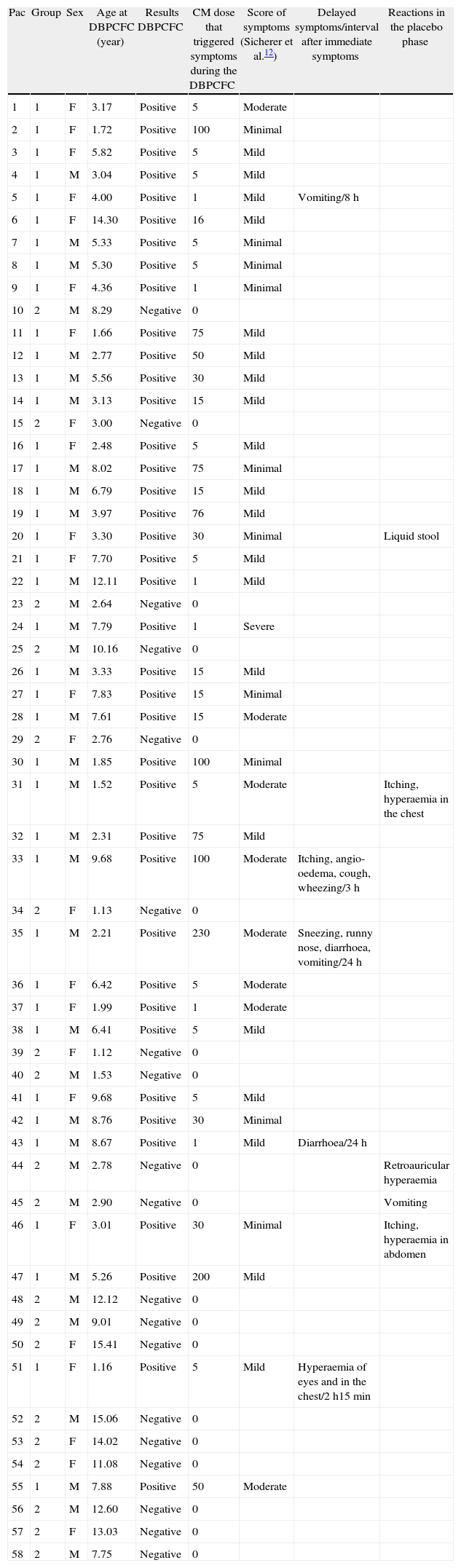
Score of symptoms and results of DBPCFC.
| Pac | Group | Sex | Age at DBPCFC (year) | Results DBPCFC | CM dose that triggered symptoms during the DBPCFC | Score of symptoms (Sicherer et al.12) | Delayed symptoms/interval after immediate symptoms | Reactions in the placebo phase |
| 1 | 1 | F | 3.17 | Positive | 5 | Moderate | ||
| 2 | 1 | F | 1.72 | Positive | 100 | Minimal | ||
| 3 | 1 | F | 5.82 | Positive | 5 | Mild | ||
| 4 | 1 | M | 3.04 | Positive | 5 | Mild | ||
| 5 | 1 | F | 4.00 | Positive | 1 | Mild | Vomiting/8h | |
| 6 | 1 | F | 14.30 | Positive | 16 | Mild | ||
| 7 | 1 | M | 5.33 | Positive | 5 | Minimal | ||
| 8 | 1 | M | 5.30 | Positive | 5 | Minimal | ||
| 9 | 1 | F | 4.36 | Positive | 1 | Minimal | ||
| 10 | 2 | M | 8.29 | Negative | 0 | |||
| 11 | 1 | F | 1.66 | Positive | 75 | Mild | ||
| 12 | 1 | M | 2.77 | Positive | 50 | Mild | ||
| 13 | 1 | M | 5.56 | Positive | 30 | Mild | ||
| 14 | 1 | M | 3.13 | Positive | 15 | Mild | ||
| 15 | 2 | F | 3.00 | Negative | 0 | |||
| 16 | 1 | F | 2.48 | Positive | 5 | Mild | ||
| 17 | 1 | M | 8.02 | Positive | 75 | Minimal | ||
| 18 | 1 | M | 6.79 | Positive | 15 | Mild | ||
| 19 | 1 | M | 3.97 | Positive | 76 | Mild | ||
| 20 | 1 | F | 3.30 | Positive | 30 | Minimal | Liquid stool | |
| 21 | 1 | F | 7.70 | Positive | 5 | Mild | ||
| 22 | 1 | M | 12.11 | Positive | 1 | Mild | ||
| 23 | 2 | M | 2.64 | Negative | 0 | |||
| 24 | 1 | M | 7.79 | Positive | 1 | Severe | ||
| 25 | 2 | M | 10.16 | Negative | 0 | |||
| 26 | 1 | M | 3.33 | Positive | 15 | Mild | ||
| 27 | 1 | F | 7.83 | Positive | 15 | Minimal | ||
| 28 | 1 | M | 7.61 | Positive | 15 | Moderate | ||
| 29 | 2 | F | 2.76 | Negative | 0 | |||
| 30 | 1 | M | 1.85 | Positive | 100 | Minimal | ||
| 31 | 1 | M | 1.52 | Positive | 5 | Moderate | Itching, hyperaemia in the chest | |
| 32 | 1 | M | 2.31 | Positive | 75 | Mild | ||
| 33 | 1 | M | 9.68 | Positive | 100 | Moderate | Itching, angio-oedema, cough, wheezing/3h | |
| 34 | 2 | F | 1.13 | Negative | 0 | |||
| 35 | 1 | M | 2.21 | Positive | 230 | Moderate | Sneezing, runny nose, diarrhoea, vomiting/24h | |
| 36 | 1 | F | 6.42 | Positive | 5 | Moderate | ||
| 37 | 1 | F | 1.99 | Positive | 1 | Moderate | ||
| 38 | 1 | M | 6.41 | Positive | 5 | Mild | ||
| 39 | 2 | F | 1.12 | Negative | 0 | |||
| 40 | 2 | M | 1.53 | Negative | 0 | |||
| 41 | 1 | F | 9.68 | Positive | 5 | Mild | ||
| 42 | 1 | M | 8.76 | Positive | 30 | Minimal | ||
| 43 | 1 | M | 8.67 | Positive | 1 | Mild | Diarrhoea/24h | |
| 44 | 2 | M | 2.78 | Negative | 0 | Retroauricular hyperaemia | ||
| 45 | 2 | M | 2.90 | Negative | 0 | Vomiting | ||
| 46 | 1 | F | 3.01 | Positive | 30 | Minimal | Itching, hyperaemia in abdomen | |
| 47 | 1 | M | 5.26 | Positive | 200 | Mild | ||
| 48 | 2 | M | 12.12 | Negative | 0 | |||
| 49 | 2 | M | 9.01 | Negative | 0 | |||
| 50 | 2 | F | 15.41 | Negative | 0 | |||
| 51 | 1 | F | 1.16 | Positive | 5 | Mild | Hyperaemia of eyes and in the chest/2h15min | |
| 52 | 2 | M | 15.06 | Negative | 0 | |||
| 53 | 2 | F | 14.02 | Negative | 0 | |||
| 54 | 2 | F | 11.08 | Negative | 0 | |||
| 55 | 1 | M | 7.88 | Positive | 50 | Moderate | ||
| 56 | 2 | M | 12.60 | Negative | 0 | |||
| 57 | 2 | F | 13.03 | Negative | 0 | |||
| 58 | 2 | M | 7.75 | Negative | 0 |
The most common clinical manifestations of patients in Group 1 (39 patients) were perioral papules (49%), oropharyngeal pruritus (44%), cough (36%), sneezing (31%), runny nose, vomiting and urticaria (22%). Some patients experienced more than one manifestation; stridor occurred in one patient, and 14 patients showed symptoms that were reported in their clinical histories in the first phase of the trial, which were considered to be positive tests. These patients were treated and released from the next phase. Nine patients underwent the two test phases and the open phase to confirm their test results. Seven patients presented subjective symptoms during the blind phase that were not referenced in their clinical histories. Only two patients exhibited symptoms (angio-oedema and hives on the lips) in the open phase. Intramuscular epinephrine was prescribed for five patients that experienced anaphylaxis during testing. Notably, none of these patients reported having anaphylaxis in the past 2years. Among these patients, four exhibited hoarseness, stridor, wheezing, hives, and angio-oedema alone or in combination. One patient exhibited pruritus in the oropharynx and rhinitis symptoms during the examination and was given epinephrine based on a previous history of anaphylaxis.
DiscussionCMA is the most common food allergy (FA) in infants and correct diagnosis is extremely important to avoid prescribing unnecessarily restrictive diets and the ensuing changes in nutritional status.13 The clinical history and presence of specific IgE are not sufficient to confirm the diagnosis. The open oral food challenges (OFC), due to its practicality, have been frequently indicated, especially in children younger than 1year with objective and immediate symptoms.4 According to Nowak-Wegrzyn et al.,14 the open OFC should be the first choice to evaluate an adverse reaction in patients with a high risk of negative outcomes. In a recent study in Brazil, Bicudo Mendonça et al. showed that the open OFC was considered suitable for children up to 3years of age in confirmation of CMA mediated by IgE.15
However, when there is risk of compromising in the interpretation of the test due to the suggestion of persons involved or when there is risk of subjective symptoms, the DBPCFC is the best indication. It is more rigorous than open OFC and due to its accuracy, it is considered to be the gold-standard method for FA diagnosis.16–18 Prior to the inception of this study, it had not been performed in our department. Because this department is a reference institution for the diagnosis and treatment of patients with FA, the development of a protocol that could be implemented for evaluating FA and a methodology that could be reproduced for other services is relevant and essential. The protocol reported here was based on the methodology described by Williams and Bock,7 which was compatible with our conditions and the characteristics of our patient population. This protocol allows the test to be performed in a single day, making it less expensive.
In this study, we opted to use a food vehicle instead of capsules, which are often used in adults. Although the capsules can be used for adolescents, their manufacturing involves costs that are not compatible with our conditions. Soy was frequently chosen because of its ability to mask the taste, odour and appearance of CM while maintaining good flavour even in small volumes. In addition, soy allows different flavours to be selected, thus improving its acceptance. Soy is also readily available in supermarkets and it can be stored.
Although many authors have described the use of an amino acid formula as a vehicle,1 this product is expensive (33 times more expensive than vegetable soup, 20 times more than the Soy-based drinks fruit Tonyu® and 13 times more than fruit juice Del Valle®) and this product has poor palatability.
The use of opaque material for the test containers was advantageous because it prevented patients from perceiving the appearance and odour of the test portions. Coloured and opaque cup holders were used to completely enclose the contents of the portions. The list of potential vehicles was expanded by using containers that concealed important characteristics of the CM, such as colour and odour. The fruit juices sold in a Tetra Pak® were used in this study with good acceptance. Beverages containing pulp or fruit juice with plenty of flavour, with natural flavourings and colourings, without proteins and sodium, and readily available in our country, were optimal options.
One of the key adjustments to the protocol described by Williams and Bock7 was related to the use of low-lactose CM during the DBPCFC. We emphasise that this need was identified during the study. The use of low-lactose CM during the DBPCFC has been described by Morisset et al.19 The adaptation of this measure dismisses the possibility of a hypolactasia diagnosis as a confounding factor.
The other adjustments made to the protocol proposed by Williams and Bock7 involved the placebo and CM doses. The authors of this study believe that the gradual increase of doses is more secure. The possibility to adjust the initial dose in accordance with the volume reported in the history is important, especially in cases with previous anaphylaxis. With regard to the time intervals between doses, especially in young children, they were extended to allow better acceptance.
A similar pattern in the clinical reactivity to that described in the literature was observed, with manifestations of oral allergy syndrome and respiratory symptoms. The former reaction has been described in association with CM20; notably, however, respiratory symptoms are rarely reported during the clinical history, while they are frequently observed during the test, particularly nasal symptoms.21
About the use of epinephrine, Bock and Atkins11 reported its use only in four of 480 patients who underwent a DBPCFC. In this study, it was used in five patients according to medical criteria: based on the severity of clinical symptoms and based on a history of previous anaphylaxis. Despite mild symptoms but with rapid evolution presented during the DBPCFC, one patient with a previous history of anaphylaxis was treated with epinephrine. Because this is a reference service, we have acquired many patients with a prior history of anaphylaxis.
This adaptation allowed to implement this important test for the diagnosis of IgE-mediated CMA in a reference centre for paediatric allergies. It was considered feasible and safe if performed in an appropriate setting with physician supervision. Future work should evaluate the reproducibility of this protocol test in other institutions and integrate it into CMA diagnosis in everyday practice.
Conflict of interestThe authors have no conflict of interest to declare.
Thanks to Dr. Cristina Kokron and Carlos R. O. Palma for carrying out the ImmunoCAP.