Atopic dermatitis (AD) is a chronic inflammatory disease of the skin. Apart from its well-known role on calcium metabolism, vitamin D is reported to affect skin functions. The study aims were to: compare the vitamin D levels of children with AD and healthy children; investigate the relationship between the severity of AD and vitamin D levels; and investigate the effect of vitamin D on the natural course of AD.
Patients or materials and methodsSixty-nine patients with AD were enrolled. Seventy healthy children were assigned as control group. Clinical and demographic features of groups were recorded. The skin prick test, eosinophil counts, immunoglobulin (Ig) E levels and serum 25 OH cholecalciferol (25OHD3) levels were measured. After at least 4 years of follow-up, patients were re-evaluated for natural course of AD.
ResultsMean 25OHD3 level was lower in patient group vs. control group; 19.86±6.7ng/mL (min–max: 6.8–40) vs. 24.07±9.08ng/mL, respectively, (p=0.002). Mean 25OHD3 levels, and vitamin D status were significantly different between AD severity groups. (p<0.05). In terms of vitamin D status in the pairwise comparison, vitamin D deficiency was greater in children with severe and moderate AD groups (respectively, p=0.005, p=0.018). In Tukey's post hoc analysis for 25OHD3 level, the 25OHD3 levels of severe AD are significantly lower than mild or moderate AD (respectively, p=0.001, p=0.026). There was a negative correlation between 25OHD3 levels and severity of AD (r=−0.480; p=0.001). In patients reassessed after 4 years: age, the age of AD onset, vitamin D deficiency, SCORAD level and severe AD were higher in the persistent group vs. remission group, 25OHD3 levels were higher in the remission group vs. persistent group (p<0.05).
ConclusionsMean vitamin D levels were lower in patients with AD. A negative correlation between vitamin D levels and disease severity was documented. Vitamin D may affect the natural course of atopic dermatitis. There is a need for more comprehensive studies in this regard.
Atopic dermatitis (AD), caused by a complex interplay of genetic and environmental factors, is a relapsing and chronic inflammatory disease of the skin. Worldwide, AD is a common health problem that affects 10–20% of children and 1–3% of adults. The exact cause of AD is not well understood, nevertheless, defective epidermal barrier function, impaired antimicrobial defence mechanisms and increased cutaneous inflammation are reported to be the major culprits.1 Defective epidermal barrier function is thought to be related to the downregulation of the filament-aggregating protein, filaggrin, reduced ceramide, loricrin, involucrin levels, increased levels of endogenous proteolytic enzymes, and enhanced trans-epidermal water loss.2
Humans obtain their vitamin D either through the skin via cutaneous conversion of 7-dehydrocholestrol into previtamin D3 by exposure to solar ultraviolet B radiation or through the gut, via food and/or supplement ingestion. Vitamin D also plays an important role in skin functions beyond acting as a hormone on calcium metabolism. It promotes cornified envelope formation and synthesis of the lipid permeability barrier.3 Antimicrobial peptides (AMPs) have a pivotal role on antimicrobial defence and are secreted at the surface of the skin as a first-line defence against infection. Liu et al.4 demonstrated that the release of AMPs (such as cathelicidin), antimicrobial response, triggered by the toll-like receptor and vitamin D-mediated immunity. The results of studies investigating the levels of vitamin D in patients with AD are contradictory. In some studies, it has been shown that there is a relationship between vitamin D levels and disease severity in patients with AD.5–9 On the contrary, there are studies showing that there is no relationship.10–12 The effect of vitamin D levels on survival of the disease has not been evaluated in children with AD.
The aims of this study were to compare the vitamin D levels of children with AD and healthy children, to investigate the relationship between the severity of AD and vitamin D levels, and to investigate the effect of vitamin D on survival of AD.
Material and methodsA total of 69 children enrolled to the study. The study was approved by the local ethics committee of the same institute and adhered to the principles of Helsinki Declaration. Consent was obtained from all subjects and/or their parents.
Study populationSixty-nine children with AD followed in Allergy-Immunology outpatient clinics of University of Health Sciences, Zeynep Kamil Woman and Children Health Practice and Research Centre were enrolled. The control group consisted of seventy children without any chronic disease. Children were evaluated in September 2012-February 2013, in order to exclude the seasonal differences. Demographic and clinical features of the study population (such as age, gender, personal/familial atopy) were recorded and a detailed history of allergic diseases was obtained. The presence of atopy in the family was considered as positive if a first-degree relative (mother, father, sibling) had allergic disease. Milk consumption of cases was not evaluated because milk is not fortified with vitamin D in our country. AD was diagnosed according to the criteria of Hanifin and Rajka.13 The severity of AD was graded by the Severity Scoring of Atopic Dermatitis (SCORAD) index. SCORAD score of <15 was classified as mild, 15–40 as moderate, and >40 as severe.14 The groups were compared in terms of clinical and laboratory findings. The patients were reassessed after at least a four-year interval. Parents of the patients who did not continue to follow up were contacted by phone and their children were evaluated in terms of whether AD continued or not. According to this assessment, the patients were divided into two groups as patients with continuing dermatitis (Persistent group) and those who recovered AD (Remission group). All patients received a skin prick test with the same allergens. The percentage of eosinophils, serum immunoglobulin (Ig) E levels and serum 25-hydroxyvitamin D3 (25OHD3) levels were measured.
Excluding criteriaPatients who had received multivitamin supplementation or any systemic glucocorticoid therapy in the previous six months, patients with obesity (body mass index >95.p), patients with the clinical findings of rickets (o-bain, x-bain, etc.), and patients with any chronic disease apart from allergic disease were excluded.
Skin prick testsSkin prick tests were applied on the anterior surface of the forearm or back when children were appropriate for test. Histamine (10mg/ml) and physiological saline were used as positive and negative controls, respectively. Skin reactions were evaluated 20min after the application of the skin test, and indurations of ≥3mm was considered indicative of a positive reaction. Skin prick tests to common aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae), cow's milk, egg yolk, egg white, potato, wheat flour, Alternaria alternata, cockroaches (Blatella germanica), and cat dander (Stallergenes SA, 92160 Antony, France) were performed using Quantitest. In patients with a history of food reactions skin prick tests were performed with the suspected food. For children older than three years of age a mixture of grass pollens (Lollium perenne, Dactylis glomerata, Phleum pratense, Anthoxanthum odaratum, Poa pratensis, Festuca eliator, Agrostis vulgaris, Holcus lanatus, Cynodon dactylon, Avena sativa, Avena fatua, Lotus Corniculatus), a mixture of grain pollens (oats, wheat, barley, corn), a mixture of tree pollens (Acer pseudoplanatus, Aesculus hippocastanum, Robinia pseudoacacia, Tilia platyphyllos, Platanus vulgaris), and weed-mix pollens (Medicago sativa, Trifolium pratense, Brassica nigra, Urtica dioica, Rumex acetosa) were added. Atopy was classified as at least one positive response to these allergens with no response to negative reference solution and a response to positive reference (histamine).
Vitamin D levelsSerum 25OHD3 levels were measured two weeks after any infection. Peripheral venous blood samples were obtained from all children and serum 25OHD3 levels were measured using liquid chromatography–tandem mass spectrometry (LC–MS–MS) method in Waters Quattro Premier XE™ (Waters Corp. Milford, USA). Serum 25OHD3 levels ≤20ng/mL were considered as vitamin D deficiency, while levels between 20 and 29ng/mL as vitamin D insufficiency, 30–80ng/mL as optimal vitamin D level and ≥80ng/mL as potential vitamin D toxicity. In the case of vitamin D deficiency, replacement therapy was given according to the guideline of the Endocrine Society Clinical Practice.15
Statistical analysesData were analysed using the Statistical Package for the Social Sciences (SPSS) Version 15.0 software (SPSS Inc., Chicago, IL, USA). Values for continuous variables were given as either mean±standard deviation or as median (interquartile range), based on the normality of distribution. Student t test, ANOVA and Kruskal Wallis test were used for the comparison of normally distributed variables. Chi-square and Mann Whitney U tests were the statistical test of choice when the distribution of continuous variables was non-normal. Pearson's and Spearman correlation tests were used for the correlation analyses of variables. p<0.05 was considered as significant.
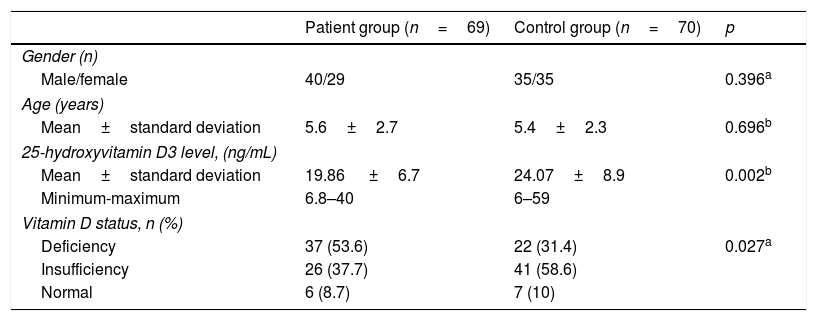
ResultsIn the patient group, forty patients (58%) were male and the mean age was 5.6±2.7 years. In the control group, thirty-five children (50%) were male and the mean age was 5.4±2.3 years. These two parameters did not differ significantly between groups (Table 1). Mean 25OHD3 level was lower in the patient group compared to the control group; 19.86±6.7ng/mL (min–max: 6.8–40) vs. 24.07±9.08ng/mL, respectively, (p=0.002). Thirty-seven children (53.6%) had 25OHD3 deficiency, 26 children (37.7%) had 25OHD3 insufficiency and six children (8.7%) had normal 25OHD3 levels in the patient group, whereas 22 children (31.4%) had 25OHD3 deficiency, 41 children (58.6%) had 25OHD3 insufficiency and seven (10%) had normal 25OHD3 levels in the control group (p=0.027) (Table 1).
Comparison of demographic characteristics and vitamin D status between patient group and control group.
| Patient group (n=69) | Control group (n=70) | p | |
|---|---|---|---|
| Gender (n) | |||
| Male/female | 40/29 | 35/35 | 0.396a |
| Age (years) | |||
| Mean±standard deviation | 5.6±2.7 | 5.4±2.3 | 0.696b |
| 25-hydroxyvitamin D3 level, (ng/mL) | |||
| Mean±standard deviation | 19.86 ±6.7 | 24.07±8.9 | 0.002b |
| Minimum-maximum | 6.8–40 | 6–59 | |
| Vitamin D status, n (%) | |||
| Deficiency | 37 (53.6) | 22 (31.4) | 0.027a |
| Insufficiency | 26 (37.7) | 41 (58.6) | |
| Normal | 6 (8.7) | 7 (10) | |
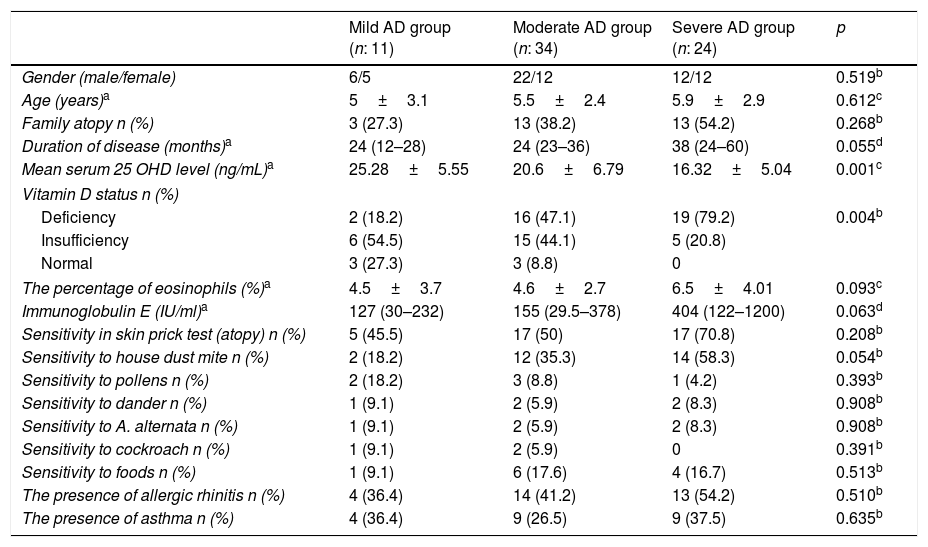
The patients were grouped into three subgroups according to their SCORAD scores, namely mild AD group, moderate AD group and severe AD group. Gender, age, the presence of familial atopy, the duration of disease, the percentage of eosinophils, IgE level, the presence of sensitivity in skin prick test, sensitivity to house dust mite, pollens, dander, A. alternata, cockroach and food did not different significantly between subgroups of patients (p>0.05) (Table 2). However, mean 25OHD3 levels, and vitamin D status were significantly different between groups (p<0.05) (Table 2). In terms of vitamin D status in the pairwise comparison of the groups, vitamin D deficiency was greater in children with severe and moderate AD groups (respectively, p=0.005, p=0.018). In Tukey's post hoc analysis for 25OHD3 level, the 25OHD3 levels of severe AD are significantly lower than mild or moderate AD (respectively, p=0.001, p=0.026). There was a negative correlation between 25OHD3 levels and severity of AD (r=−0.480; p=0.001).
Comparison of demographic, clinical features and laboratory findings of the patients among subgroups.
| Mild AD group (n: 11) | Moderate AD group (n: 34) | Severe AD group (n: 24) | p | |
|---|---|---|---|---|
| Gender (male/female) | 6/5 | 22/12 | 12/12 | 0.519b |
| Age (years)a | 5±3.1 | 5.5±2.4 | 5.9±2.9 | 0.612c |
| Family atopy n (%) | 3 (27.3) | 13 (38.2) | 13 (54.2) | 0.268b |
| Duration of disease (months)a | 24 (12–28) | 24 (23–36) | 38 (24–60) | 0.055d |
| Mean serum 25 OHD level (ng/mL)a | 25.28±5.55 | 20.6±6.79 | 16.32±5.04 | 0.001c |
| Vitamin D status n (%) | ||||
| Deficiency | 2 (18.2) | 16 (47.1) | 19 (79.2) | 0.004b |
| Insufficiency | 6 (54.5) | 15 (44.1) | 5 (20.8) | |
| Normal | 3 (27.3) | 3 (8.8) | 0 | |
| The percentage of eosinophils (%)a | 4.5±3.7 | 4.6±2.7 | 6.5±4.01 | 0.093c |
| Immunoglobulin E (IU/ml)a | 127 (30–232) | 155 (29.5–378) | 404 (122–1200) | 0.063d |
| Sensitivity in skin prick test (atopy) n (%) | 5 (45.5) | 17 (50) | 17 (70.8) | 0.208b |
| Sensitivity to house dust mite n (%) | 2 (18.2) | 12 (35.3) | 14 (58.3) | 0.054b |
| Sensitivity to pollens n (%) | 2 (18.2) | 3 (8.8) | 1 (4.2) | 0.393b |
| Sensitivity to dander n (%) | 1 (9.1) | 2 (5.9) | 2 (8.3) | 0.908b |
| Sensitivity to A. alternata n (%) | 1 (9.1) | 2 (5.9) | 2 (8.3) | 0.908b |
| Sensitivity to cockroach n (%) | 1 (9.1) | 2 (5.9) | 0 | 0.391b |
| Sensitivity to foods n (%) | 1 (9.1) | 6 (17.6) | 4 (16.7) | 0.513b |
| The presence of allergic rhinitis n (%) | 4 (36.4) | 14 (41.2) | 13 (54.2) | 0.510b |
| The presence of asthma n (%) | 4 (36.4) | 9 (26.5) | 9 (37.5) | 0.635b |
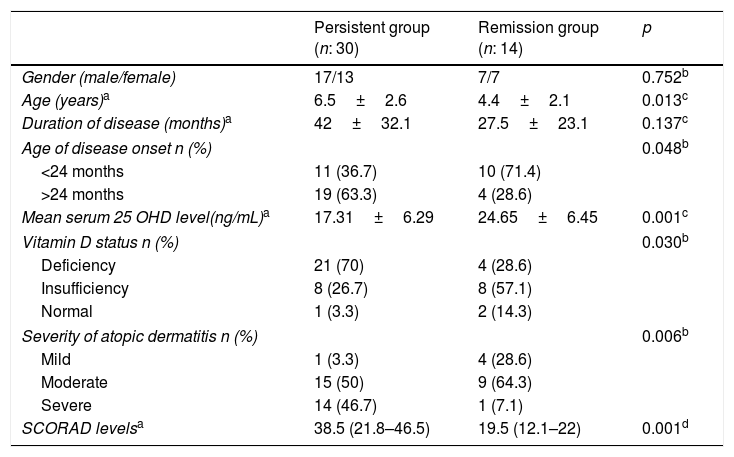
Follow-up visits were planned between 3 and 12 months. After/at least four years following the first examination, patients were reassessed as to whether atopic dermatitis continued or not. Fifteen patients continued on follow-up. 29 patients’ parents were interviewed by telephone. A total of 44 patients were re-evaluated. The remaining 25 patients could not be evaluated because they did not interview for various reasons, such as incorrect phone numbers, unrecorded phone number, and poor parental understanding. Atopic dermatitis persisted in 30 (68.2%) of these 44 patients. Patients were divided into two groups as those with persistent atopic dermatitis and those with complete remission. There was no significant difference in terms of gender, familial atopy, presence of asthma or allergic rhinitis, sensitivity in skin prick tests, sensitivity to allergens and vitamin D insufficiency between these groups (p>0.05) (data not shown). However, while age, the age of AD onset, vitamin D deficiency, SCORAD level and severe AD were higher in the persistent group compared to the remission group, 25OHD3 levels were higher in the remission group compared to the persistent group (p<0.05) (Table 3).
Comparison of demographic, clinical features and laboratory findings of the patients between persistent group and remission group of atopic dermatitis.
| Persistent group (n: 30) | Remission group (n: 14) | p | |
|---|---|---|---|
| Gender (male/female) | 17/13 | 7/7 | 0.752b |
| Age (years)a | 6.5±2.6 | 4.4±2.1 | 0.013c |
| Duration of disease (months)a | 42±32.1 | 27.5±23.1 | 0.137c |
| Age of disease onset n (%) | 0.048b | ||
| <24 months | 11 (36.7) | 10 (71.4) | |
| >24 months | 19 (63.3) | 4 (28.6) | |
| Mean serum 25 OHD level(ng/mL)a | 17.31±6.29 | 24.65±6.45 | 0.001c |
| Vitamin D status n (%) | 0.030b | ||
| Deficiency | 21 (70) | 4 (28.6) | |
| Insufficiency | 8 (26.7) | 8 (57.1) | |
| Normal | 1 (3.3) | 2 (14.3) | |
| Severity of atopic dermatitis n (%) | 0.006b | ||
| Mild | 1 (3.3) | 4 (28.6) | |
| Moderate | 15 (50) | 9 (64.3) | |
| Severe | 14 (46.7) | 1 (7.1) | |
| SCORAD levelsa | 38.5 (21.8–46.5) | 19.5 (12.1–22) | 0.001d |
We demonstrated that serum 25 OHD levels were significantly lower in patients with AD compared to healthy controls. It is reported that vitamin D has a crucial role in skin functions and has vital importance in the maintenance of antimicrobial defence mechanisms and epidermal barrier function. For this reason, it is not implausible to expect an association between vitamin D deficiency and an increased AD incidence. Research investigating the relationship between vitamin D level and AD disease severity revealed controversial results. Studies demonstrating a correlation between serum vitamin D levels and disease severity in patients with AD (5–9) and studies documenting no correlation at all10–12 are both present in literature. In a new meta-analysis assessing vitamin D levels in AD, seven observational studies were evaluated. As a result, Vitamin D levels especially in childhood atopic dermatitis were found to be lower than controls.16 Our result is consistent with this meta-analysis.
In our study, we determined that 25OHD3 levels were lower in patients with severe AD compared to patients with mild and moderate AD. Vitamin D has been shown to have an important role in AMP function. AMPs are secreted by many different cells in the skin, including keratinocytes and mast cells. Human cathelicidin, a chemoattractant for neutrophils and monocytes, enhances microbial killing in phagocytic vacuoles and has a defined vitamin D-dependent mechanism.17 Peroni et al.7 reported an increased susceptibility to infections with Staphylococcus aureus and M. Furfur in AD patients with vitamin D insufficiency.7 Vitamin D deficiency leads to low levels of AMP and impairment of skin barrier functions thus rendering the individual prone to infections. As a result of them, recurrent infections increase the severity of AD by enhancing the inflammation of skin. Peroni et al.7 have reported that mean serum 25(OH)D levels were significantly higher in patients with mild AD (36.9±15.7ng/mL) compared to those with moderate (27.5±8.3ng/mL) or severe AD (20.5±5.9ng/mL) (p<0.05). Akan et al.5 reported a negative correlation between disease severity and serum vitamin D level in AD patients with allergen sensitivity. Lee et al.8 documented a correlation between vitamin D levels and AD disease severity only in patients with food allergy. In our study, we detected a negative correlation between vitamin D levels and AD disease severity in patients with AD. In contrast to these studies, Samochocki et al.10 conducted a study in an adult population and failed to document a connection between vitamin D level and SCORAD scores. Similarly, Chiu et al.11 could not demonstrate a link between serum vitamin D levels and AD disease severity. These inconsistent results may be attributed to cross-sectional nature of these studies and to clinical, demographic and seasonal discrepancies of the study populations.
Another important result of our study is that the current age, age at onset of disease, severity of atopic dermatitis and vitamin D deficiency were higher in the persistent group compared to the remission group. Factors which affect the persistence of atopic dermatitis are examined in a new meta-analysis. In this meta-analysis, the onset of atopic dermatitis after two years of age, the duration of illness, the severity of AD at baseline and female gender were found as factors affecting the persistence of atopic dermatitis.18 Consistent with the meta-analysis, we also found that the onset of the disease after two years and severe AD were more in the persistent group compared to remission group in our study. While the duration of disease was higher in the persistent group, this difference was not statistically significant. Similarly, we also found no difference in terms of gender. The inconsistency in our results may have occurred due to the small number of the sample.
Interestingly, we found that children with atopic dermatitis with vitamin D deficiency are more likely to have disease persistence. This relationship has not been investigated before in the literature. Comprehensive studies are needed in order to make a clearer decision on this issue.
A major limitation of our study is its small sample size. It is a single-centre study which only recruited children. Our cross-sectional study investigated vitamin D levels which were obtained once in autumn or winter period. More than one sample could have been obtained and mean vitamin D levels throughout the year could have been measured in order to minimise the seasonal differences. Despite these limitations, our study is important in terms of patients being followed-up by the same allergist at the same health centre and showing the relationship between vitamin D level and the natural history/progress of atopic dermatitis, which have not been evaluated previously in the literature.
As a result, we found that 25OHD3 levels in atopic dermatitis were lower than in controls. In addition, a negative correlation between severity of AD and vitamin D level was detected. After at least four years of follow-up, age, the onset of the disease after two years of age, severe AD and vitamin D deficiency in the persistent group were higher than in the remission group. Vitamin D levels must be measured in patients with severe AD and replacement therapy should be performed in case of deficiency. There is a need for more comprehensive studies on the effect of vitamin D levels on the natural course of AD.
Data access and responsibilityThe principal investigator, Mahmut Dogru, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial supportThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe author declares that they have no conflict of interest.
None declared.