Specific immunotherapy (SIT) is used to treat asthma and allergic rhinitis, and a dose–response relationship has been found for SIT efficacy, creating a need to accurately select the allergen used in therapy. This need is especially pronounced in poly-sensitized children living in areas where different pollen allergen sources coexist in the same season, as this circumstance complicates diagnostic efforts. In such cases, component-resolved diagnosis (CRD) can increase diagnostic accuracy and aid in SIT prescription.
Materials and MethodsWe hypothesized that CRD results would lead to modifications in classical immunotherapy prescription based on sources such as medical history, season of symptom presentation, and skin testing. We studied a sample of children indicated for immunotherapy in whom classical methods had not pointed out the most relevant allergen due to sensitization to more than two pollens. We used a small panel of recombinant allergens, analyzing the percentage of changes to prescription considering the findings of molecular studies.
ResultsOf the 70 children included, CRD led to modified immunotherapy prescription in 54.3%. Indications of single-allergen therapy increased from 18% to 51% when CRD was included. The decision to prescribe immunotherapy was reversed following CRD in 9.3% of cases.
DiscussionCRD use alters the choice of specific immunotherapy in poly-sensitized children. A wide panel of recombinant allergens may not be necessary to improve immunotherapy indication using molecular techniques; rather, a smaller panel adapted to include those allergens prevalent in the geographical area in question appears to be sufficient for more effective immunotherapy, also leading to an improved cost–benefit ratio.
Allergen-specific immunotherapy (SIT) is commonly used to treat asthma and allergic rhinitis, and is the only treatment that can modify the natural course of the disease.1 Single-allergen studies have proved the superior efficacy of SIT compared to placebo,1–3 although only low-grade evidence has been gathered from studies using multiple allergens,4,5 both in patients with asthma or with rhinitis. The European Academy of Allergy and Clinical Immunology (EAACI) recommends the use of extracts containing a low number of allergen sources, as higher doses of extracts with few allergens appear to show greater improvement, demonstrating a dose–response relationship for clinical efficacy in immunotherapy.3,6 However, in areas where several pollens coexist during the same season (as occurs in Madrid during the spring season), it is a challenge for clinicians to distinguish between genuine IgE sensitization and cross-reactivity in multi-sensitized patients, thus complicating efforts to recognize true allergy-causing agents and impeding correct etiological diagnosis.7
Component-resolved diagnosis (CRD), also called molecular diagnosis, or purified natural allergens, has recently been introduced in clinical allergy practice and may improve not only diagnostic accuracy but also immunotherapy prescription, facilitating the choice of the most relevant allergens in each patient.7
Although multi-sensitization is frequently associated with the adult population, this phenomenon is increasingly seen in children, adding to the difficulty of identifying the true relevant allergens using classical diagnostic methods based on clinical history and skin prick test (SPT).
Based on this existing evidence, we hypothesized that purified allergen molecules can improve SIT prescription in daily practice.
Materials and methodsThe aim of this study was to determine whether use of CRD in lieu of classical diagnostic methods modifies the indication and pollen prescription of SIT in multi-sensitized children in whom both clinical history and SPT fail to identify the most relevant pollen-allergen source.
We carried out a descriptive study under routine conditions of clinical practice. Of the 182 patients who met the clinical criteria for SIT indication and had positive SPT results, the relevant allergen was identified using traditional methods in 106 cases (58%), and as a result CRD was not performed. The intervention group (n=76) included only those children with clinical indication of SIT found to be sensitized to more than two pollens by SPT, in whom CRD was performed due to the inability of classical diagnostic methods to indicate the most appropriate extract. Of these, patients with incomplete data on both SPT and CRD were excluded (n=6).
The study was approved by the research ethics committee of the hospital. Informed consent was not requested, as in all cases patients were managed in accordance with routine clinical practice.
A skin prick test (SPT) was performed with a panel of standardized allergen extracts (ALK-Abelló, Madrid, Spain) including the following pollens: Grass mix, Cynodon dactylon, Phragmites communis, Olea europea, Cupressus arizonica, Platanus acerifolia, and Plantago lanceolata. Other non-pollen allergens such as Dermatophagoides (pteronyssinus and farinae), Aternaria, and pet dander were also studied. Results were evaluated according to the criteria recommended by the EAACI.8 For all patients, we measured the specific IgE antibody levels for Phl p 1, Phl p 5, Phl p 7, Phl p 12, Ole e 1, and Cup a 1 (Immunocap, Phadia, Uppsala, Sweden). At the time the study was conducted, Pla a1 and Pla l1 were not available.
Only one physician was involved in the clinical part of the study. Before receiving the CRD results, SIT was prescribed based on clinical history, the time of year in which symptoms occurred, and SPT results; this information (called SIT A here) was recorded in the patient medical records. Once the results of the molecular diagnosis were available, the same physician reanalyzed the prescription, making changes where appropriate (SIT B). SIT B was the treatment the patient ultimately received.
The patient characteristics studied were sex and age (median and interquartile ranges), SPT and CRD results, asthma and rhinoconjunctivitis severity, and allergen prescription (A and B). Determinations were considered to be in disagreement if a discrepancy in the indication and allergen composition was found between SIT A and B, that is, if the study physician modified the SIT prescription in light of the findings of molecular diagnosis. Data were analyzed by frequency distribution and were performed using SPSS version 15.0 (Chicago, IL, USA).
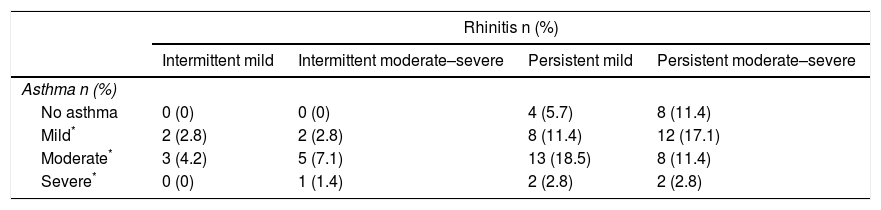
ResultsSeventy (70) children (71.4% male) met the criteria and were included in the study. Median age was 10 years (interquartile range, 8–12 years). The distribution of patients based on guidelines for assessing the severity of rhinoconjunctivitis (ARIA)9 and asthma (GINA)10 was as follows: 24 patients with mild asthma underwent immunotherapy, most of whom presented persistent rhinitis (83%); 72% of the 29 patients with moderate asthma had associated persistent rhinitis; and in five patients with severe asthma, immunotherapy was prescribed, presenting rhinitis in varying degrees. Twelve patients (17%) were prescribed immunotherapy due to persistent rhinitis, although with no associated asthma, while no patients presenting intermittent rhinitis but without asthma were administered immunotherapy (Table 1).
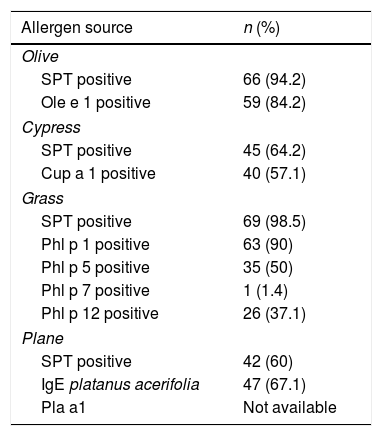
Table 2 shows the sensitization pattern of patients to all pollens analyzed. In addition, 36 patients were sensitized to other inhaled allergens, such as molds, dust mites, or animal danders. Where Phl p1 and/or 5 produced mostly positive results, the patient was considered to be truly sensitized to grass-pollen allergens, while positive test results to Phl p7 and 12 were considered to be evidence of cross-reactivity. Ole e1 and Cup a1 are considered to be the major allergens of their respective sources.
Allergen sensitization as revealed by SPT and CRD.
| Allergen source | n (%) |
|---|---|
| Olive | |
| SPT positive | 66 (94.2) |
| Ole e 1 positive | 59 (84.2) |
| Cypress | |
| SPT positive | 45 (64.2) |
| Cup a 1 positive | 40 (57.1) |
| Grass | |
| SPT positive | 69 (98.5) |
| Phl p 1 positive | 63 (90) |
| Phl p 5 positive | 35 (50) |
| Phl p 7 positive | 1 (1.4) |
| Phl p 12 positive | 26 (37.1) |
| Plane | |
| SPT positive | 42 (60) |
| IgE platanus acerifolia | 47 (67.1) |
| Pla a1 | Not available |
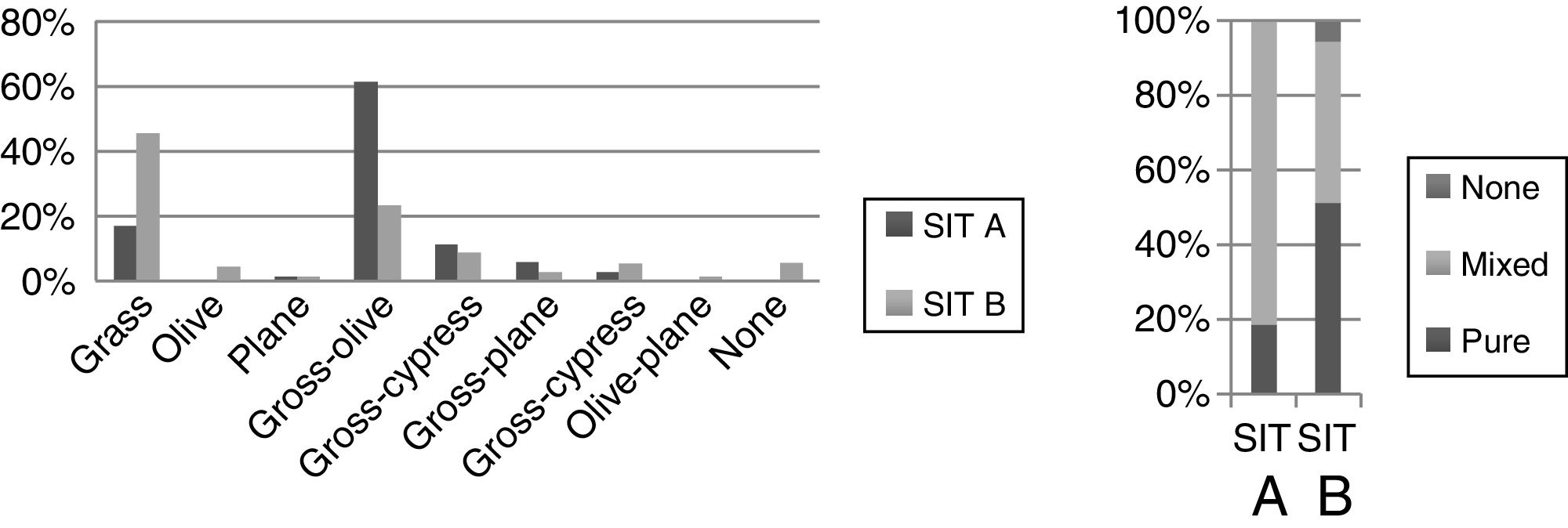
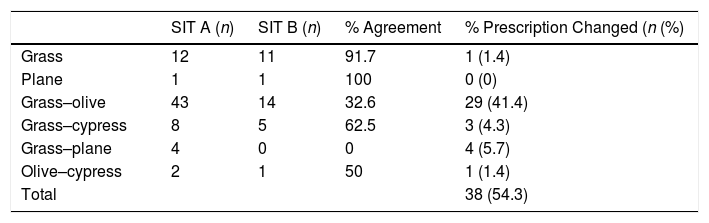
Concerning the prescribed immunotherapy, as mentioned previously, SIT A was based on traditional diagnostic methods, while SIT B factored in the results of recombinant allergens. An analysis of the pollen composition revealed that the grass–olive combination was the most common initial prescription in SIT A, while the pure grass extracts were administered more than any other (SIT B) (Table 3). Furthermore, in those patients in whom the initial decision was to prescribe a grass–olive combination SIT, this prescription was maintained in only 32.6% of cases once the component diagnosis was analyzed, while modification to pure grass extract occurred in 44.2% and to olive extract in 4.7% of the children studied (Table 4). However, when the initial decision was to administer the grass mix only, this decision was maintained in 91.7% of patients. Very few combinations other than grass or olive were used, thus precluding any statistically significant results. Overall, we observed that when using CRD, single-allergen immunotherapy prescription increased from 18% when classical methods were used to 51% when the findings of molecular diagnosis were taken into account. In another important finding, no SIT whatsoever was indicated in 9.3% of cases once CRD was carried out due to the low sensitization to major allergens and the presence of panallergens (Tables 3 and 4).
Percentage of agreement between both immunotherapy types for each prescription.
| SIT A (n) | SIT B (n) | % Agreement | % Prescription Changed (n (%) | |
|---|---|---|---|---|
| Grass | 12 | 11 | 91.7 | 1 (1.4) |
| Plane | 1 | 1 | 100 | 0 (0) |
| Grass–olive | 43 | 14 | 32.6 | 29 (41.4) |
| Grass–cypress | 8 | 5 | 62.5 | 3 (4.3) |
| Grass–plane | 4 | 0 | 0 | 4 (5.7) |
| Olive–cypress | 2 | 1 | 50 | 1 (1.4) |
| Total | 38 (54.3) |
The percentage of discrepancies between SIT A and B was 54.3%, meaning no change in prescription was required in only 45.7% of cases, while 54.3% of the prescribed immunotherapies were modified. The greatest percentage of modifications was made in cases in which mixed allergens were initially prescribed for immunotherapy (Table 4).
DiscussionThis study shows that the use of CRD in clinical practice may not only modify SIT prescription (54.3%), but also increased prescriptions made up of pure extract, thus leading to better immunotherapy, as stated by EAACI.6
Years ago, a similar study was carried out in an adult population in our geographical area. The study reported that 54% of the SIT prescriptions were modified using CRD.11 Although multi-sensitization is thought to occur more frequently in adult patients, and CRD might not be that useful in children, we have found a similar percentage of change in SIT prescription in our study.
Another study performed in a large sample of Italian children concluded that up to 47% of SIT prescriptions can be modified based on CRD not only in clinical practice but also applying different theoretical models on prescription indications of immunotherapy.12 Similar results (56.8% of SIT-prescription modifications) were obtained in a study carried out in areas of our country with a high prevalence of coexisting grasses and olive tree sensitization,13 and other authors have also reported changes in SIT prescription according to CRD results.14
The discrepancy observed between prescriptions before and after CRD may be secondary to a cross-reactivity phenomenon caused by panallergens and not true sensitization. Indeed, skin prick extracts contain mixtures of various allergens, some of which are specific for the allergen source, while others contain cross-reactive allergens from various unrelated sources.11,13
Regarding extract composition, most studies analyzing the impact of CRD have observed a decrease in the use of allergen mixtures in immunotherapy and an increase in single-allergen SIT, as shown in our study.13 The issue of whether polyallergic patients are most optimally treated with several allergens or one allergen (the most clinically relevant) remains a concern among physicians. Although allergen-mixture immunotherapy may be effective, some authors have concluded that those with more than two allergens should be investigated further.5 Some highlights the lack of prescription after learning the result of CRD, which occurs in up to 20.9% of cases13; this figure is higher than that obtained in our study.
This study has certain limitations, including the scant number of patients included (n
=70) compared to other publications. Additionally, not all patients with SIT indication were included, but rather only those multi-sensitized children in whom classical diagnostic methods did not allow the most relevant allergen to be identified. As a result, we have not been able to completely identify the sensitization pattern in our patients or establish the most widely used extracts in our practice. Despite this limitation, the fact that the present study was carried out in routine clinical practice is a source of strength, especially as CRD is not requested in all patients assessed, and is reserved to those polysensitized children with no clear choice of extract. Rather than using CRD as an alternative, we believe molecular methods should be used in conjunction with less costly, traditional specific IgE tests in selected patients, as other authors do.15 Another limitation of this study is that we have not analyzed the whole recombinant panel described in the literature, limiting our study to Phl p 1, Phl p 5, Phl p 7, Phl p 12, Ole e 1, and Cup a 1. Currently, pla a1 is also requested, although this was not the case at the time of the study. Again, this aspect also lends certain strength to our study, as it appears that a small panel may be sufficient to complement classical diagnostic methods, providing an adequate cost–benefit ratio.
It should not be forgotten that better prescribed immunotherapy will avoid inclusion of allergens that are irrelevant for the patient or diluting those which are relevant, and such optimized SIT prescription will lead to more effective treatment.2
Conflict of interestThe authors have no conflict of interest to declare.
We thank Oliver Shaw for editorial assistance.