In the past few decades, the prevalence of allergic diseases has deeply increased, with a key role played by food allergies. Legumes seem to play a major role towards the overall increase in the scenario of food allergy, since they are an appreciated source, consumed worldwide, due to their high protein content, variable amounts of lipids and for the presence of vitamins.
In literature there are numerous descriptions of adverse reactions after ingestion of uncooked and cooked legumes. Nevertheless, cases of allergic reactions induced by inhaling vapours from cooking legumes have rarely been described.
Herein the authors report an update of the literature data on allergic reactions caused by legume steam inhalation, underlying the possible pathogenic mechanism of these atopic events and the knowledge of literature data in paediatric age. The importance of this review is the focus on the clinical aspects concerning legume vapour allergy, referring to literature data in childhood.
In the past few decades, the prevalence of allergic diseases has deeply increased, with a key role played by food allergies.1,2 Acute reaction to food allergens is a fairly common problem both in childhood and in adulthood, its incidence being especially high in childhood, with a range between 1.4 and 4%.1,2 Basically, food allergies, defined as adverse reactions to an otherwise harmless food protein, due to an abnormal reaction of the immune system towards these proteins that are recognised as foreign elements. Consequently the immune system triggers a response to neutralise them.3
Anaphylaxis consequent to the consumption of an allergenic food affects a significant proportion of the population. In fact its prevalence is about 6–8% in children and 4% in adults.4–6 Over 90% of food allergies are caused by eight main food groups that are: peanuts, soybeans, cow's milk, hen's egg, fish, crustacean, wheat, and tree nuts. The reaction can be severe in some cases and the severity of food-induced anaphylaxis was described by Wang and Sampson,7 who mentioned that in the U.S.A. about 30,000 persons are treated for food anaphylaxis in the emergency department per year. Peanuts, tree nuts, fish and shellfish were mostly responsible for these anaphylactic reactions.
Legumes seem to play a major role towards the overall increase in the scenario of food allergy, because they are a worldwide-appreciated source due to their high protein content, variable amounts of lipids and vitamins.
Legumes are dicotyledonous plants belonging to the Fabales order, which is composed by four families: Mimosaceae, Caesalpiniaceae, Papillonaceae and Fabaceae. White bean and green bean (Phaseolus vulgaris) are members of the Fabaceae family and few cases of systemic reactions in children after their ingestion or vapours inhalation have been reported.8 Among these legumes, lentils, belonging to the Papillonaceae family, seem to be the most common legumes implicated in paediatric allergic reactions in the Mediterranean area and India.9–12 In the literature there are numerous descriptions of adverse reactions after ingestion of uncooked and cooked legumes: oropharyngeal symptoms and acute urticaria are the most common symptoms, followed by anaphylaxis.9–12 Nevertheless, cases of allergic reactions induced by inhaling vapours from cooking legumes have rarely been described, both in adulthood and in childhood.
Herein the authors report an update of the literature data in childhood on allergic reactions caused by legume steam inhalation, underlying the possible pathogenic mechanism. The importance of this review is the focus on the clinical aspects reported in the literature concerning legumes allergy, referring to literature data in paediatric age.
The prevalence of legume allergy in different countriesIn a vegetarian diet, legumes are the main source of proteins. However, these crops have been described to be responsible for IgE-mediated reactions in Mediterranean and Asian countries.3 As far as the prevalence of legume allergies in the Western countries is concerned, peanut allergy is common in the UK, France, Switzerland, and North America, whereas higher prevalence of soybean allergy is found in Japan.3 Lupine is another legume extensively consumed in Mediterranean countries. The prevalence of sensitisation to this legume varies. In a recent study of 1160 patients in the Mediterranean area, a lupine sensitisation rate of 4.1% was reported among atopic patients.13 Prevalence of lupine allergy in France and Belgium was reported to present a cross-reactivity sensitisation to peanut,14 with a rate of 14.5% of adults and 17% of children allergic to peanut and cross-reacting to lupines. In Denmark, sensitisation to lupine was found in 82% of 39 patients allergic to peanut.15 Chickpea, red gram and mung bean are some of the crops playing a major role for food allergies in the Indian population.3,16 Lentils and chickpeas have been reported to cause IgE-mediated sensitisations – particularly in paediatric patients.3
This variation of allergenic condition from country to country seems to be linked to different cultural and dietary habits, and an increased consumption of a specific crop may lead to sensitisation against that particular crop.3 However, other factors, as a result of genetic factors and/or exposure to new allergenic proteins early in life may influence the prevalence variation of legume allergy from a country to another one.
The allergic pathophysiology of lentil steam atopyAlthough most food atopic reactions occur after ingestion of the responsible food, by IgE-mediated allergic reactions, few cases may occur through the exposure to airborne food allergen particles.17
The large legume family (Fabaceae) comprises 730 genera with over 19,400 species, including important agricultural crops like peanuts, beans, peas, soy, lentils, chickpeas and lupins. An increasing number of legume proteins have been found to be allergenic. Peanut, soy and lupin are among the major food allergens with relevance for the public health.18
The rising prevalence19 and seriousness of peanut allergy20 has led to a corresponding increase in studies evaluating the allergenic potentials of the individual peanut proteins, including members of four dominant plant allergen families.19,20 Peanut profilin (Ara h 5), pathogenesis-related (PR-10) pollen protein (Ara h 8), prolamins (Ara h 2, Ara h 6, Ara h 7, Ara h 9), cupins (Ara h 1, Ara h 3, Ara h 4) and oleosins (Ara h 10, Ara h 11) have been molecularly characterised and immunochemically studied.21–24
As far as lentils are concerned, two different types of allergens have been characterised from boiled lentils. The first type consists of three proteins named len c1 and len c2 of 16kD, and a third protein called len c3 of 12kD, belonging to the vicillin protein family and having a structural and immunochemical relationship. These proteins represent the main IgE-binding group in boiled lentil extracts. Among them, len c1 in lentils allergy was first described for its relevance, due to its high percentage of recognition in individual sera of patients affected by lentil allergy (68%). It has also been demonstrated by commercial lentil CAPs that len c1 has an IgE-binding inhibitory capacity (64%).17,25–27
Another mechanism responsible for lentil allergic reactions is referred to the hypersensitivity caused by their steam inhalation, even if in the literature few data are reported on this regard. In 1992, Martin et al.10 described the case of a 20-year-old man who experienced asthmatic attacks after exposure to the steams from cooking chickpea and lentils. Type I hypersensitivity to the antigens of these legumes was demonstrated by means of immediate skin reactivity, radioallergosorbent test (RAST), RAST inhibition and histamine release tests. The authors10 identified a protein band with intense specific IgE binding in the homologous boiled legume extracts of lentil, chickpea and pea. They stated that this allergen is thermostable and remains active in cooked legumes. It was also showed that a number of extremely resistant immunoreactive proteins still remained even after autoclaving. Later, in 2000, Sanchez-Monge et al.28 sought to purify and characterise the relevant IgE-binding proteins from boiled lentil extracts via the sera of patients who had allergic reactions after lentil ingestion. They studied boiled lentils, assuming that they were ingested after having been heated and this probably caused inactivation and/or modification of the putative allergens. The authors found a high similarity between one of the most allergenic protein components of boiled lentils and pea provicillins. Following this research line, later Vereda et al.29 identified the IgE-binding-epitopes of len c1, demonstrating that lenc1 is involved in lentil steam atopy, even if the pathophysiological pathway leading to the phenotypic expression still remains unknown. According to these data, it seems that lenc1 is a heat stable protein and when inhaled it is responsible for atopic reactions in sensitised patients. These allergic reactions seem to be referable to IgE-mediated hypersensitivities in hosts previously sensitised to food by ingestion.30,31 Thus, the host experiences clinical manifestations also after simple inhalation of boiling food vapours, similarly to what happens for inhalants allergy.32
Recently a new pathogenic hypothesis on the onset of lentil vapour atopy seems to highlight the involvement of Bruchus lentis, a lentil pest. Armentia et al.33 reported 16 patients, aged between 10 and 40 years old, who presented allergic symptoms related to inhalation or ingestion of boiled lentils, in which sensitisation to legume proteins was not clear (diagnostic tests with pure lentil extracts were found negative in vivo and in vitro). These patients showed a positive result for RAST and Prick tests for protein antigen extracts from B. lentis. These results were found negative for lentils when an accurate pest control for B. lentis was previously performed.
Accurate diagnosis of lentils and legume allergy is challenging and essential. Measurement of IgE response to specific allergenic molecules may be more useful in predicting the presence and severity of clinical allergy than currently used skin or blood tests based on whole extracts. However, given the heterogeneity in component recognition patterns observed in different geographical areas, further studies are essential to identify and confirm potentially useful molecular diagnostic and prognostic markers. Martínez Alonso et al.,8 suggested that lentil extracts for the diagnosis of lentil hypersensitivity should be heated, since boiled extracts, used at a concentration of 0.5 or 5mg/mL, best identify clinically sensitive individuals. The heating process causes a significant decrease in specific IgE binding. On the contrary, IgE-inhibition studies34 showed that the boiled lentil extracts have a greater inhibitory capacity than the crude extracts and immunoblots do not reveal important differences in IgE-binding patterns between the two extracts.34
The importance of thermal processing in legume allergiesProcessing of food may cause the transformation of one form of food into another form that is safer and more nutritious. Moreover it may enhance the self-life of food as well.35
In literature it has been demonstrated that different processing methods, such as physical, chemical and biochemical ones, may affect the allergenicity of food depending on the nature of a particular food.36,37 Food processing methods may enhance, decrease or sometimes have no effect on the allergenic potential of food. Processing may lead to the formation of entirely new allergens, known as neoallergens, responsible for enhancing the allergenicity.35,38
As far as the allergenicity of food crops is concerned, food protein is hydrolysed by enzymatic hydrolysis as a result of sequential action of endoproteases (alcalasse) and exoproteases (flavourzyme) to decrease the allergenic potential.35,39 Thermal treatment is considered the cheapest and easiest way for reducing the allergenicity of food proteins, involving the use of high temperatures that kill microorganisms and inactive enzymes to ensure the safety of food. However complete elimination of allergens from food is not possible through food processing and a combination of various processing methods can be used together as a better option. In this regard, Kasera et al.40 led a study to investigate the effect of different processing methods on the allergenicity of legume proteins. The extracts were processed by boiling, γ-irradiation or by a combination of both. They found that thermal processing resulted in a 3- to 4-fold reduction in soluble protein. Specific IgE binding of soluble protein of kidney bean, black gram and peanut respectively was reduced after boiling, with a statistically significant difference (p<0.01), whereas there was no statistically significant difference in IgE-binding reduction in the insoluble protein fraction of respective legumes. Boiling followed by γ-irradiation reduced IgE binding significantly (p<0.05). Biopotency of soluble protein of kidney bean, black gram and peanut was reduced 7-, 3- and 26-folds (p<0.001) respectively, and that of insoluble protein decreased 6-, 4- and 8-folds (p<0.001), respectively, after boiling. Combination treatment was effective in reducing the potency of both soluble and insoluble proteins significantly as compared to boiling alone (p<0.001). However, γ-irradiation alone did not bring any change in allergenicity and only boiling before γ-irradiation was effective in attenuating allergenicity of legume proteins.
Another study on the impact of thermal processing on the allergenicity of food crops was headed by Vissers et al.41 who aimed to establish the effect of heating and glycation on the IgE-binding properties and biological activity of 2S albumins (Ara h 2/6) from peanut. Ara h 2 and 6 were also purified from roasted peanut. Using peripheral blood mononuclear cells (PBMC) and sera from peanut-allergic patients, the authors assessed the cellular proliferative potency and IgE reactivity (reverse EAST inhibition) and functionality (basophile degranulation capacity) of allergens. Extensive denaturation, hydrolysis and aggregation of the protein were observed after heating Ara h 2/6 at 110°C, whilst Ara h 2 and 6 isolated from roasted peanut retained its native conformation. The thermal processing did not affect the allergen stimulation of PBMC-induced proliferation and Th2 cytokine secretion. Conversely, IgE reactivity and functionality of Ara h 2/6 was decreased by heating. Whilst heating glycation further reduced the IgE-binding capacity of these proteins, it moderated their loss of histamine releasing capacity. Ara h 2 and 6 purified from roasted peanut demonstrated the same IgE reactivity as unheated, native Ara h 2/6.
Achieving model processing conditions to ensure that thermal modifications can be monitored by structural and immunological analysis is difficult since heating frequently renders much of the protein insoluble. This makes purification of proteins from cooked foods, such as roasted or boiled peanuts, cumbersome.42 In the study of Vissers et al. the analysed proteins only began to unfold following heating at temperatures over 100°C, conditions which equated to boiling for extended times (longer than 15min). After this time the protein adopted a random coil conformation and formed dimers and higher order oligomers, accompanied by hydrolysis of the peptide backbone. Such effects of thermal denaturation have been observed for other food proteins, including the lipid transfer protein from barley which shares the same protein scaffold as the 2S albumins.43 Interestingly, the Ara h 2 and 6 purified from roasted peanuts had retained the structural characteristics of the native protein purified from raw peanuts, as indicated by far-UV CD spectra and non-denaturing electrophoresis. In addition, proteins from roasted peanut have a similar IgE immunoreactivity as the unheated protein. These data demonstrated that the proportion of the protein in roasted peanut that was still soluble and then extractable had not been denatured by the roasting process, probably due to protection within the peanut seed. A similar pathogenic mechanism could be also attributed to the thermal processing of other kind of legumes.
Lentil atopy and cross-reactivitySeveral authors have described the presence of cross-reactivity among different legumes and between legumes and various vegetables, probably due to profilins and lipid transfer proteins.44 Ibañez et al. demonstrated that peanut allergy can be associated with lentil, chick-pea and pea allergy, but white bean and overall green bean are well tolerated by children allergic to other legumes. Pea and bean are the legumes with the highst rate of in vitro cross-reactivity with Lolium perenne, Olea europea and Betula alba.35 It is possible to consider cross-reactivity between legumes of the same family by an idiosyncratic pathogenic mechanism. In this regard, the literature data show that the proteins with allergenic properties that are more frequently responsible for these reactions are vicillins.17 They are trimeric proteins of 150–190kD without disulphide bonds. Their subunit composition varies among legume species for the differences in the posttranslational processing, by proteolysis and glycosylation reactions on the initially synthesised polypeptide chains of around 50 kd.17 However, Bernhisel-Broadbent and Sampson demonstrated that clinically important cross-reactivity to legumes in children is very rare.45
Other cross-reactivity reactions have been reported, and in this regard allergy to grass pollen has been described to be associated with peanut allergy and may reflect cross-reactivity between food allergens and pollen allergens.46
Literature data and case reports on legume vapours allergy described in childhoodReports on allergic reactions to legumes have been seldom reported. This food has been associated with occupational asthma in a homemaker during preparation and cooking of raw green beans,47 with asthma and rhinitis induced by exposure to raw green beans and chards,48 and with rhinoconjunctivitis and acute asthma associated with green beans.49
Although the vast majority of IgE-mediated allergic reactions to foods occur through ingestion, literature data have described few cases of unexpected allergic reactions to foods after the exposure to airborne food allergen particles. Up to now, the majority of the cases who were reported to suffer immediate hypersensitivity reactions, such as rhinoconjunctivitis and asthma exacerbations, with the inhalation of lentil and other legumes have been adults.50 Reported cases of hypersensitivity reactions resulting from inhalation of steam containing soy and peanut are more common than those for other legumes.51
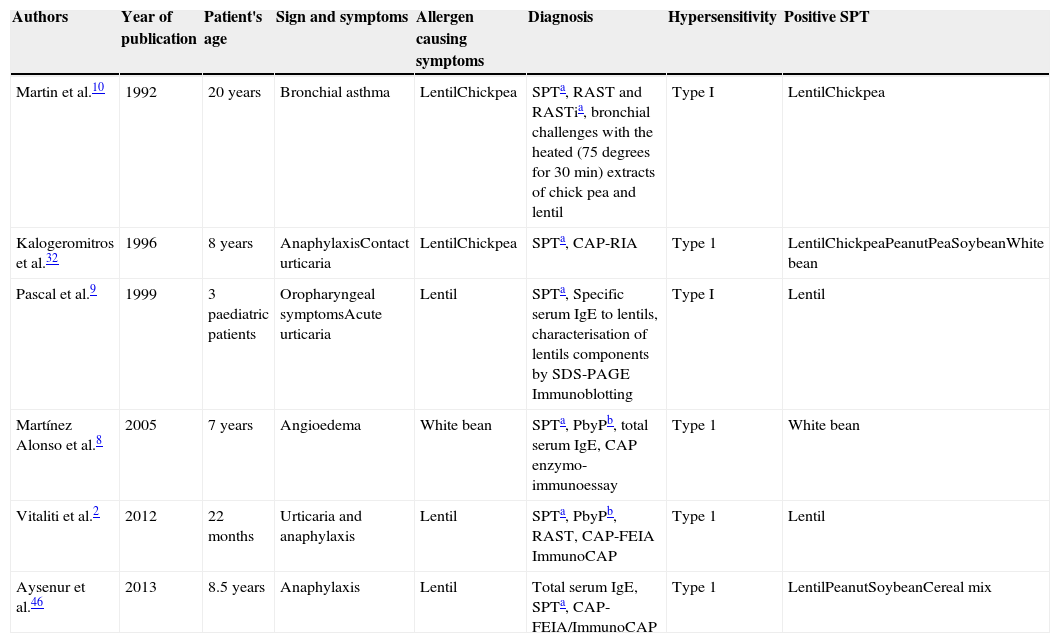
In childhood there are few reports on legume vapours atopic reactions, nevertheless, all these reports suggest the presence of hypersensitivity to aeroallergen particles coming from boiling legumes (Table 1). In this regard, in 1992, Martin et al. firstly reported a case of bronchial asthma induced by chick pea and lentil steam in a 20-year-old man,10 proposing a type I hypersensitivity as pathogenic mechanism. Later, in 1996, Kalogeromitros et al. described an eight-year-old girl with repeated anaphylactic reactions related to lentils after ingestion of cooked lentils and one episode with inhalation exposure to cooking lentil soup,32 as expression of secondary hypersensitivity. In 1999, three paediatric patients were reported to have developed urticaria, oral allergy syndrome and allergic rhinitis after exposure to steam from cooked chickpeas and green beans.9 Another report on atopic reactions after legume steam inhalation was reported by Martinez Alonso et al., in 2005, who described a non-atopic seven-year-old boy with two episodes of angio-oedema occurred after ingestion and during cooking of white beans. Type I hypersensitivity to the white beans antigens was demonstrated by means of skin tests, specific IgE determination by CAP, and the oral challenge test corroborated it.8 Recently, in 2012, Vitaliti et al.2 reported a case similar to that described by Kalogeromitros et al., with the onset of urticaria and anaphylaxis after inhalation of lentils vapours, secondary to a previous sensitisation to ingested lentils. The child was a 22-month-old child and she was the youngest child with this kind of atopic reactions ever described in literature. The last report on anaphylaxis after lentil inhalation was recently described by Aysenur et al., who presented an eight-year-old child who had anaphylactic and milder allergic reactions after ingestion of boiled forms of lentil, pea and chickpea, and who developed anaphylaxis after boiling lentil steam inhalation.46
Main features of the patients described in the literature affected by allergic reactions to legumes steam inhalation.
| Authors | Year of publication | Patient's age | Sign and symptoms | Allergen causing symptoms | Diagnosis | Hypersensitivity | Positive SPT |
|---|---|---|---|---|---|---|---|
| Martin et al.10 | 1992 | 20 years | Bronchial asthma | LentilChickpea | SPTa, RAST and RASTia, bronchial challenges with the heated (75 degrees for 30min) extracts of chick pea and lentil | Type I | LentilChickpea |
| Kalogeromitros et al.32 | 1996 | 8 years | AnaphylaxisContact urticaria | LentilChickpea | SPTa, CAP-RIA | Type 1 | LentilChickpeaPeanutPeaSoybeanWhite bean |
| Pascal et al.9 | 1999 | 3 paediatric patients | Oropharyngeal symptomsAcute urticaria | Lentil | SPTa, Specific serum IgE to lentils, characterisation of lentils components by SDS-PAGE Immunoblotting | Type I | Lentil |
| Martínez Alonso et al.8 | 2005 | 7 years | Angioedema | White bean | SPTa, PbyPb, total serum IgE, CAP enzymo-immunoessay | Type 1 | White bean |
| Vitaliti et al.2 | 2012 | 22 months | Urticaria and anaphylaxis | Lentil | SPTa, PbyPb, RAST, CAP-FEIA ImmunoCAP | Type 1 | Lentil |
| Aysenur et al.46 | 2013 | 8.5 years | Anaphylaxis | Lentil | Total serum IgE, SPTa, CAP-FEIA/ImmunoCAP | Type 1 | LentilPeanutSoybeanCereal mix |
a SPT: Skin Prick Test.
According to these literature data, there is a wide range of phenotypical expression of legume steam allergy, with symptoms referring to bronchial asthma, anaphylaxis, urticaria and angio-oedema. In some cases cross reactivity is evident, as symptoms were mostly caused by one or two main allergens, but diagnostic tests, such as SPT and serum specific IgE were positive for a higher number of allergens in most cases. All the reports refer to a type I hypersensitivity, even if challenge tests with the heated extracts of the implicated allergens would be helpful for the diagnosis, and only in the report of Martin et al.10 Nevertheless, as Aysenur et al.46 observed, a challenge test in patients who experienced anaphylaxis would have been dangerous, above all if other less invasive diagnostic tests already gave a positive result.
All the reported data are the expression of a secondary sensitisation to inhaled vapours of legumes after a first reaction due to their ingestion. It could be speculated that some protein particles of legumes are processed during the boiling procedure and then vehicled as inhaled particles to the respiratory mucosa, leading to such atopic reactions. Nevertheless, as there are few data on allergic sensitisation to food steams, further studies have to clarify the pathogenic mechanism of such allergic reactions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.