Clarithromycin or azithromycin is not infrequently used as empiric treatment for upper respiratory tract infections. When patients develop a rash after several days of treatment, one is often faced with the diagnostic dilemma of whether this is a drug eruption or viral exanthema. Most times, if the temporal sequence is in keeping with a drug eruption, this patient will be peremptorily labelled with a macrolide allergy as the literature has little to guide us through a diagnostic pathway for macrolide allergies.
Macrolides are characterised by a large lactone ring, which makes up the main structure, and can vary from 12 to 16 atoms. Although commonly thought of as a class of antibiotics, there are also non-antibiotic macrolides such as tacrolimus and sirolimus.1 Macrolide antibiotics are used for a wide variety of infections, but are particularly useful for upper respiratory tract infections2. They are recommended by the British Thoracic Society as a first-line treatment together with amoxicillin for hospitalised patients with moderate to severe community acquired pneumonia, and are also an important component of the treatment regimen of Helicobacter pylori (HP) infection.3,4
It would be wrong to deny a patient the future use of such a useful drug when the aetiology of the rash might be infectious or due to another concurrent drug. However, unlike the case of beta-lactams, skin prick tests and intra-dermal tests for macrolide antibiotics have not been widely performed and validated, and some of the macrolide antibiotics are not available in injectable forms amenable for skin testing. Lymphocyte transformation tests and histamine release tests for the diagnosis of macrolide allergy have been reported, but give inconsistent results.5
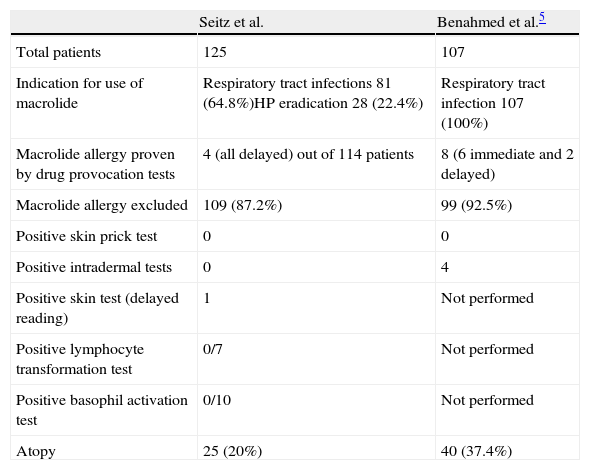
In this issue of Allergologia et Immunopathologia, Seitz et al. examine the results of allergy testing in a large cohort of patients (n=125) with suspected macrolide allergy and prove that 87.2% of patients in their series could have been unnecessarily labelled as allergic to macrolides and hence disallowed appropriate medication or an effective treatment regimen for HP eradication. So what lessons can we learn from this study?
History and physical examination may not always yield the answerHistory and physical examination have been held to be paramount in reaching any medical diagnosis, and even more so in allergology where a meticulous drug history is essential. In this study, we learn that despite a high probability based on history alone, only four of 125 patients actually had a macrolide allergy after allergy tests. Furthermore, one of these patients was initially documented to have a maculopapular rash but developed a fixed drug eruption on provocation.
These results mirror those in an earlier large study by Benahmed et al. where only eight of 107 patients suspected to have macrolide allergy were confirmed to have a true allergy (Table 1). In that series too, the description of the initial rash was sometimes not the rash produced by drug provocation.6
Evaluation of suspected macrolide allergy in 2 large studies were able to exclude a true allergy in a vast majority of patients.
| Seitz et al. | Benahmed et al.5 | |
| Total patients | 125 | 107 |
| Indication for use of macrolide | Respiratory tract infections 81 (64.8%)HP eradication 28 (22.4%) | Respiratory tract infection 107 (100%) |
| Macrolide allergy proven by drug provocation tests | 4 (all delayed) out of 114 patients | 8 (6 immediate and 2 delayed) |
| Macrolide allergy excluded | 109 (87.2%) | 99 (92.5%) |
| Positive skin prick test | 0 | 0 |
| Positive intradermal tests | 0 | 4 |
| Positive skin test (delayed reading) | 1 | Not performed |
| Positive lymphocyte transformation test | 0/7 | Not performed |
| Positive basophil activation test | 0/10 | Not performed |
| Atopy | 25 (20%) | 40 (37.4%) |
Interestingly, Seitz et al. also looked at a subgroup of patients who received a macrolide as part of the treatment of HP eradication who underwent additional skin and challenge tests to the other drugs used in the regimen, and found that a drug cause for the rash could be definitely excluded in 15 out of 28 patients. This highlights the fact that despite a close temporal relationship with medication intake, a significant number of patients may develop rash from other non-drug related causes.
Skin tests do not have a high yield in the evaluation of macrolide allergyDifferent types of skin tests can be used based on the likely mechanism of the drug reaction—skin prick tests and intradermal tests for immediate, IgE mediated reactions; or late-reading intradermal and possibly patch tests for delayed reactions. These have been extensively used and validated for beta-lactam allergies.7
Although skin prick tests, intradermal tests and patch tests have been used in the evaluation of macrolide allergies, they tend to yield positive results in 25–50% of patients tested.5 In this study, skin tests (appropriate to corresponding clinical symptoms) were negative except for a positive delayed reaction in a single patient (25%). Skin tests alone would have missed 75% of the proven allergies in this series.
Lymphocyte transformation test and basophil histamine release test are not validatedThe lymphocyte transformation test and basophil histamine release test have been described in a few cases of macrolide allergies with conflicting results; some cases gave positive results while others had negative results despite positive drug provocation tests. None of the tests done in this series were positive and more studies are needed to help us come to a conclusion about the usefulness of these tests.
Drug provocation tests remain the key to diagnosing macrolide allergyDrug provocation tests are considered the gold standard to exclude or confirm the diagnosis of a drug hypersensitivity reaction. However, they cannot be performed in patients with severe life-threatening reactions such as Stevens–Johnson syndrome or toxic necrolysis, and are contraindicated in some patients with other medical conditions like pregnancy and severe asthma or cardiac disease where a provoked reaction might cause serious harm to the patient.8
Both this series and the earlier one by Benahmed et al. have shown that drug provocation tests exclude macrolide allergy in the vast majority of suspected patients. Another study by Messaad et al. showed that only 13.7% of 102 patients with suspected macrolide allergy had confirmed reactions on drug provocation tests.9 None of the reactions following provocation tests were severe or could not be controlled easily with medications. Not performing drug provocation tests for macrolide allergy would grossly overestimate the true incidence of macrolide allergy by almost 10-fold.
Hence, history and physical examination, skin and in vitro tests do not appear to be very helpful in disproving or confirming the diagnosis of macrolide allergies. Perhaps it is time that we consider performing drug provocation tests on all patients who have a history suggestive of macrolide allergy and no contraindications to provocation tests, as these have been shown to be generally safe with careful patient selection.