Macrolides are useful in a wide range of bacterial infections including upper and lower respiratory tract, skin, and sexually transmitted diseases and are used in Helicobacter pylori eradication regimen. Skin symptoms occurring during drug therapy are mostly attributed to the antibiotic, causing considerable limitations of future therapeutic options. The aim of this retrospective analysis was to demonstrate results of diagnostic testing in cases of clinically suspected immediate and delayed macrolide hypersensitivity.
MethodsA total of 125 patients with a history of immediate or delayed hypersensitivity symptoms in temporal relation to treatment with a macrolide antibiotic were studied using standardised skin tests followed by oral challenges. Selected patients with severe symptoms were further evaluated with in vitro tests.
Results. Macrolide hypersensitivity was excluded in 109 patients (87.2%) by tolerated oral challenge tests. During 113 challenges in four patients an exanthema was provoked by the suspected macrolide. Only one patient developed a positive late skin test reaction. Out of the 28 Helicobacter pylori-treated patients, one patient with clarithromycin allergy was identified, whereas in eight cases amoxicillin allergy caused the exanthema. Laboratory tests using the suspected macrolides were constantly negative.
ConclusionsHistory alone leads to an over-estimation of macrolide hypersensitivity. Moreover, skin and in vitro tests seem to be not very useful in identifying hypersensitive patients. Challenge tests appear to be necessary for definitely confirming or ruling out macrolide allergy.
Macrolides, which are structurally characterised by their lactonic cycle structure, are effective antibiotics against gram positive and gram negative bacteria.1 They may be indicated for upper and lower respiratory tract infection, skin and soft tissue infection or sexually transmitted diseases. Clarithromycin is preferentially used in the eradication therapy of Helicobacter pylori (HP) infection. Macrolides are considered to be one of the safest antibiotics in clinical practice with few adverse reactions, most commonly affecting the gastrointestinal tract with clinical symptoms such as nausea, cramping, diarrhoea, or rarely pseudomembranous colitis.2 Less common events include liver enzyme abnormalities, prolongation of the QT interval, and transient ototoxicity. Besides directly drug-related side effects, immediate and delayed hypersensitivity reactions to macrolides have been observed.3 Urticaria accounts for the majority of reported reactions but maculo-papular exanthemata, fixed drug eruption, and bullous skin reactions have also been reported.4–6 However, previously published case series are of limited significance because macrolide allergy diagnosis relied only on a suggestive history without allergological diagnostic evaluation.7–9
Helicobacter pylori is associated with various gastro-duodenal diseases such as peptic ulcer, functional dyspepsia, MALT lymphoma, and distal gastric cancer. First-line therapy consists of a 7-day treatment regimen with a proton pump inhibitor (PPI) in combination with clarithromycin and amoxicillin or metronidazole, respectively.10 Symptoms of immediate or delayed hypersensitivity developing during this treatment regimen or shortly thereafter are usually attributed either to the macrolide clarithromycin, or amoxicillin, metronidazole, and the PPI, and may have considerable impact on future prescription of these compounds. Therefore, in these cases allergological testing is of utmost importance to establish a correct diagnosis and to prevent an unjustified label of drug allergy concerning several drug classes.
The aim of this retrospective analysis was to evaluate the reliability of diagnostic allergological procedures including skin, in vitro, and oral challenge testing for definite identification or exclusion of macrolide hypersensitivity.
Materials and MethodsPatientsFrom 2000 to 2009, all patients referred to our allergy clinic with a history suggestive of a macrolide-induced hypersensitivity reaction were retrospectively identified. After a thorough review of patient files all available clinical data were collected. The reported anaphylaxis symptoms were classified according to severity as described.11 Extent of exanthema was graded as mild (grade 1=macular or maculo-papular eruption,<25% body surface area), moderate (grade 2=macular or maculo-papular eruption, 25 to 50% body surface area), and severe (grade 3=macular, papular or pustular eruption, covering > 50% body surface area); severe bullous skin reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis were not observed. As part of the standard practice in our allergy clinic all subjects had been informed about any risks involved with testing and written informed consent for allergological work-up (skin tests, in vitro tests, oral challenge) had been obtained. Since determination of potential drug allergy is part of routine diagnostic practice in our clinic, further ethical approval was not required.
Skin testsIn patients with immediate reactions we performed prick and intradermal tests on the volar forearm with reading after 20minutes, according to international standards.12 For prick testing macrolide tablets (500mg erythromycin, 250mg clarithromycin, 50mg roxithromycin, 250mg azithromycin) were ground in a mortar and suspended with 1mL physiological saline solution. Prick testing was done through this suspension dropped on the volar forearm. For intradermal testing available parenteral macrolide preparations, i.e. erythromycin, clarithromycin, and azithromycin, were diluted to 0.01mg/mL. All agents were freshly reconstituted, and physiological saline solution was used as negative control. In patients with delayed hypersensitivity symptoms additional patch tests on the upper back were performed at least six weeks after clearance of the skin rash. For patch testing Finn-chambers with an inside diameter of 8mm and height of 0.4mm were filled with approximately 20 to 30μL of the same suspension as prepared for prick testing. Patches were removed after one day and for late reactions patch, intradermal, and prick test sides were evaluated after two, three and four days. In individual cases hypersensitivity to drugs administered concomitantly with the macrolides were excluded as potential triggers of the hypersensitivity symptoms by additional skin and challenge tests, e.g. PPIs; other antimicrobial drugs such as amoxicillin or metronidazole; and non-steroidal anti-inflammatory drugs, as described previously.13,14
Laboratory testsIn selected cases with severe symptoms (anaphylaxis ≥ grade 2, exanthema grade 3) additional laboratory tests were performed. The basophil activation test is based on the drug-induced specific activation of basophils and was performed in 10 patients as described previously.15 The lymphocyte transformation test measuring the proliferation of T cells to a drug was carried out in seven patients.16 For tryptase determination in 15 anaphylaxis patients commercially available ImmunoCAP™ Tryptase (a test for the quantitative measurement of tryptase concentration in human serum) was used.17
Oral challengePatients were offered oral challenge tests according to an established protocol using standardised macrolide doses: erythromycin 62.5; 125; 250; 500mg; clarithromycin 62.5; 125; 250; 500mg; roxithromycin, 12.5; 25; 50; 150mg, and azithromycin 62.5; 125; 250mg, respectively. In children, dosage of macrolides was age/weight-adjusted. The general principles of our challenge protocol were as follows14,18: (i) the time interval since the hypersensitivity reaction was at least six weeks; (ii) during the entire challenge procedure the patient was observed and equipment for emergency treatment was available; (iii) the dosage of the macrolide increased stepwise to a normal dose with intervals of one hour between the individual doses; (iv) strict adherence to absolute and relative contraindications for drug challenge tests; and (v) prior to challenge testing written informed consent was obtained from each patient.
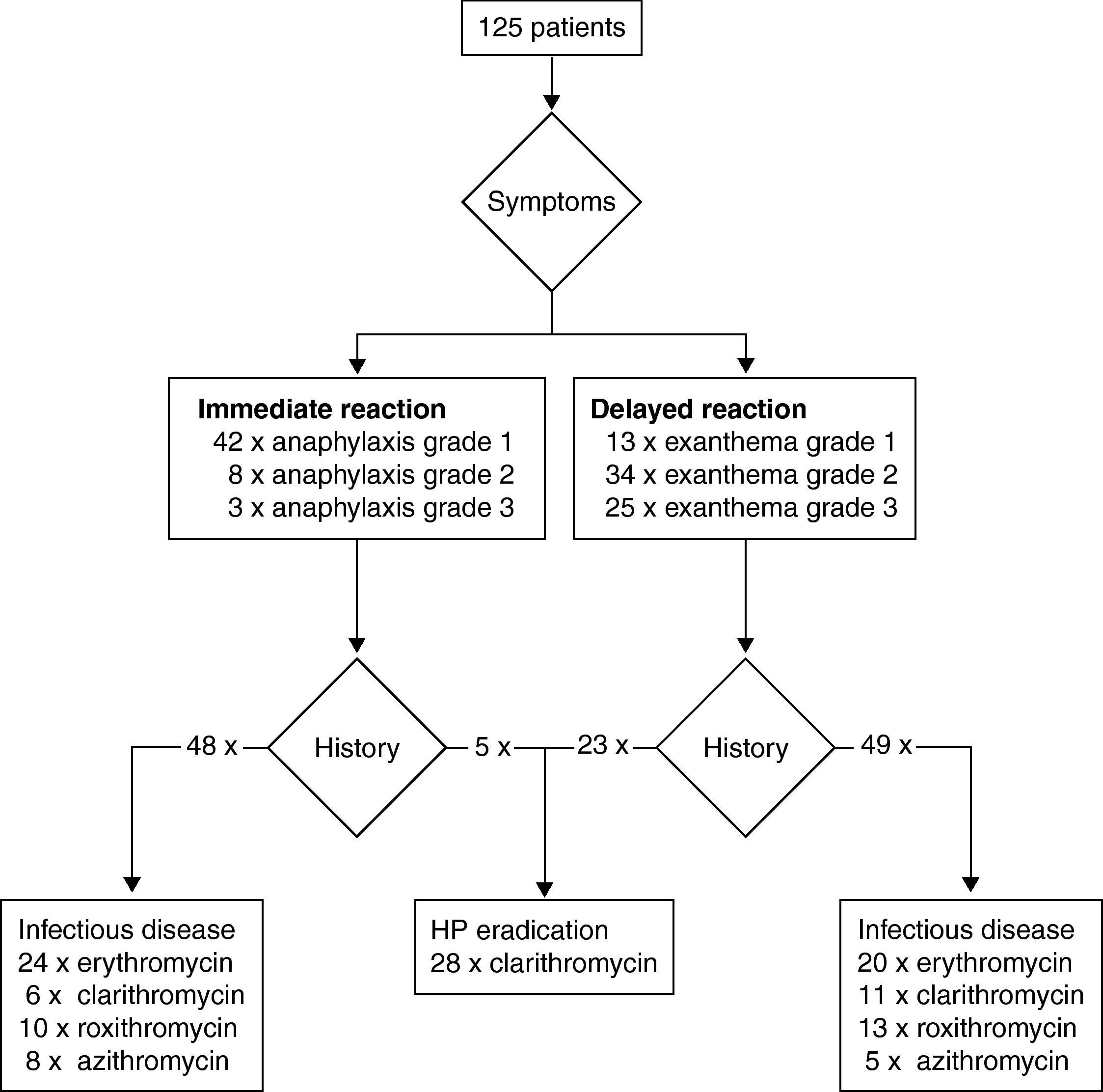
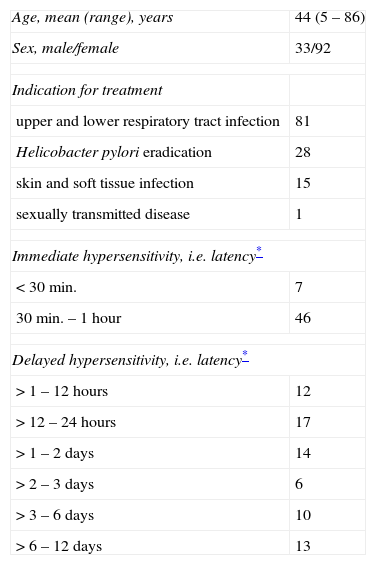
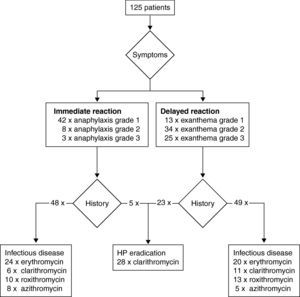
ResultsPatientsA total of 125 patients were included in the analysis. All patients were referred by health care professionals, i.e. general practitioners, consultants, or allergologists suspecting macrolide hypersensitivity. The mean age of the subjects (92 females and 33 males) at the time of the suspected macrolide-induced hypersensitivity reaction was 44 years (ranging from 5 to 86 years). Twenty-five patients (20.0%) were considered atopic due to a positive skin prick test with aeroallergens or a history of atopic dermatitis, rhinitis or asthma. In the vast majority of cases the macrolide had been administered orally (n=121), in four patients intravenously. In Table 1 clinical data including underlying infectious disease and latency of the reaction (i.e. time interval between start of treatment and onset of symptoms) are summarised. Depending on the latency between administration of the macrolide and clinical symptoms we classified the reactions as immediate in 53 patients, and as delayed in 72 patients. Fig. 1 shows type and severity of symptoms and the incriminated macrolides. The time interval between the suspected hypersensitivity reaction and allergological work-up was less than one year in 97 cases, in the remaining 28 it was in the range of one to two years.
Clinical data of the 125 patients studied.
| Age, mean (range), years | 44 (5 – 86) |
| Sex, male/female | 33/92 |
| Indication for treatment | |
| upper and lower respiratory tract infection | 81 |
| Helicobacter pylori eradication | 28 |
| skin and soft tissue infection | 15 |
| sexually transmitted disease | 1 |
| Immediate hypersensitivity, i.e. latency* | |
| < 30min. | 7 |
| 30min. – 1 hour | 46 |
| Delayed hypersensitivity, i.e. latency* | |
| > 1 – 12 hours | 12 |
| > 12 – 24 hours | 17 |
| > 1 – 2 days | 14 |
| > 2 – 3 days | 6 |
| > 3 – 6 days | 10 |
| > 6 – 12 days | 13 |
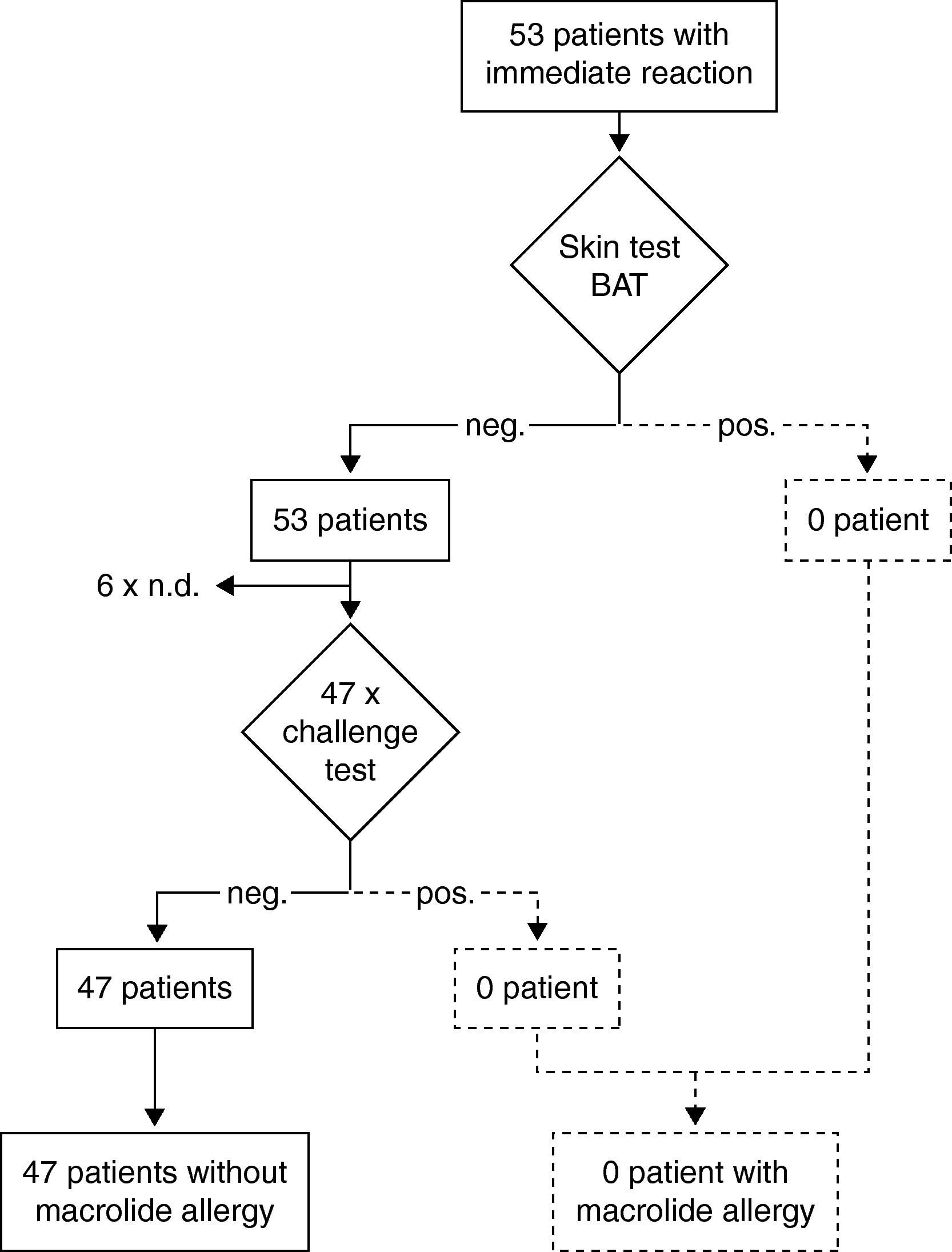
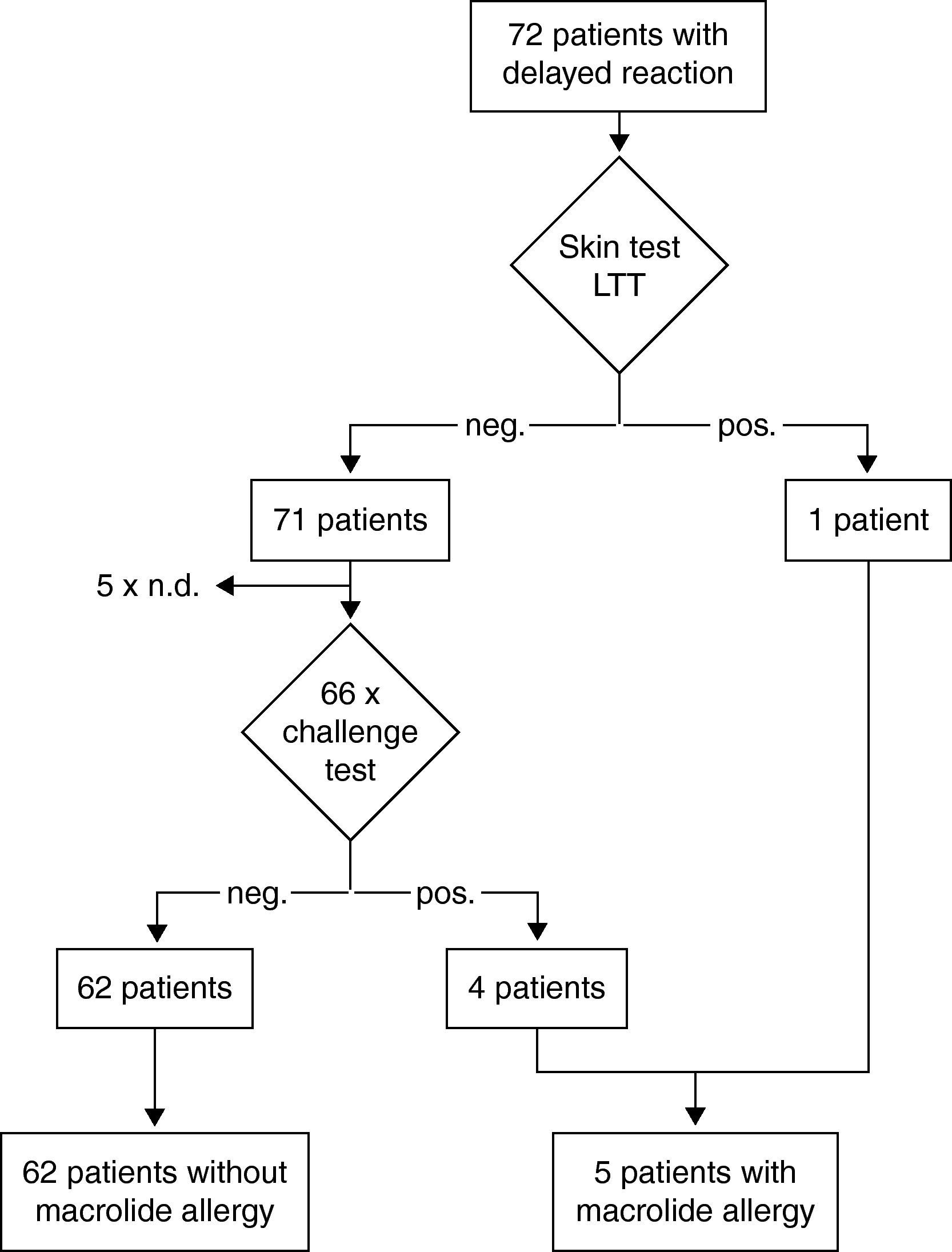
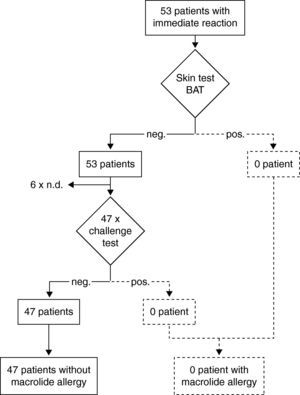
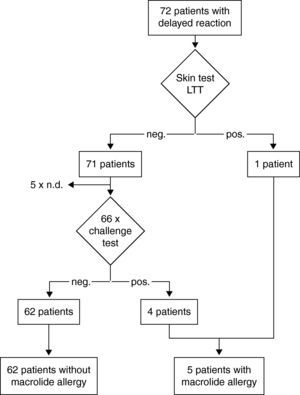
Results of the diagnostic procedure are summarised in two separate flow charts: one for immediate and one for delayed reactions (Figs. 2 and 3). Skin tests were applied corresponding to the clinical symptoms, i.e. immediate reading of prick and intradermal tests after 20minutes in patients with immediate reactions and delayed reading of prick, intradermal and patch tests in delayed reactions, respectively. All skin tests in patients with immediate reactions were negative (Fig. 2), whereas a positive delayed skin test reaction to roxithromycin was observed in one patient (Fig. 3).
Steps and results of the allergological work-up in 53 patients with immediate reactions in temporal relation to macrolide treatment. neg.; negative result. pos.; positive result. Diagnosis in case of a positive skin or challenge test is depicted with dotted lines because this has not occurred in this study. BAT; basophil activation test.
With the suspected macrolide neither basophil activation tests performed in 10 patients nor lymphocyte transformation tests performed in seven patients yielded positive results. In one patient out of 15 (including the 11 reporting with anaphylaxis ≥ grade 2) the tryptase serum level measured six weeks after anaphylaxis was elevated with 42.2 ng/mL (normal range < 11.4 ng/mL).
Oral challenge(A) Immediate hypersensitivity. Forty-seven subjects out of 53 with a history of an immediate reaction were negatively challenged with the suspected macrolide (Fig. 2). Three patients refused challenge tests and in three patients challenge testing was contra-indicated due to underlying severe cardiovascular disease or malignancy. (B) Delayed hypersensitivity. Four of 66 challenged patients developed maculo-papular exanthema (Fig. 3, Table 2). Five patients refused challenge tests. In summary, macrolide hypersensitivity could be definitively excluded in 109 patients by performing the described allergological work-up.
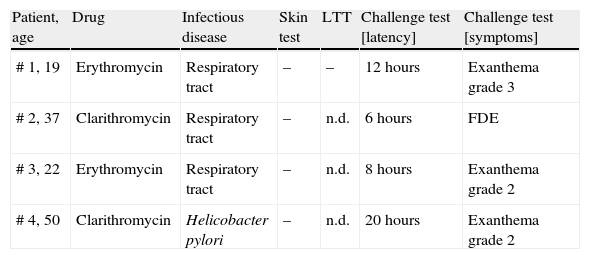
Results of skin and challenge tests in 4 patients with proven macrolide hypersensitivity.
| Patient, age | Drug | Infectious disease | Skin test | LTT | Challenge test [latency] | Challenge test [symptoms] |
| # 1, 19 | Erythromycin | Respiratory tract | – | – | 12 hours | Exanthema grade 3 |
| # 2, 37 | Clarithromycin | Respiratory tract | – | n.d. | 6 hours | FDE |
| # 3, 22 | Erythromycin | Respiratory tract | – | n.d. | 8 hours | Exanthema grade 2 |
| # 4, 50 | Clarithromycin | Helicobacter pylori | – | n.d. | 20 hours | Exanthema grade 2 |
n.d.; not done. –; negative result. For the grading system for exanthema see Materials and Methods. LTT; lymphocyte transformation test. FDE; fixed drug eruption.
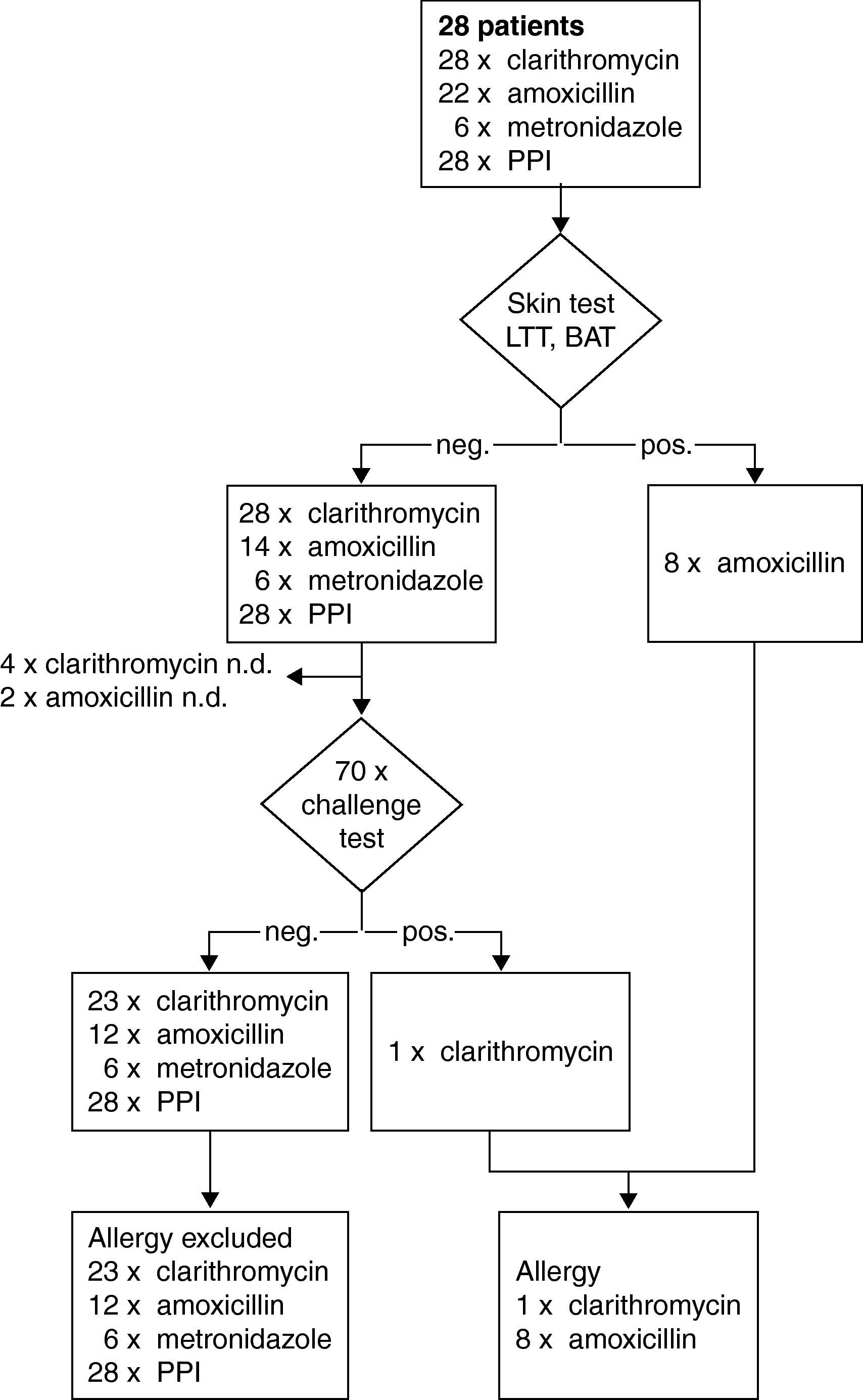
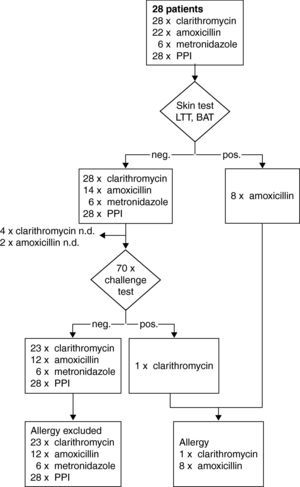
A subset of 28 patients was identified in the study with suspected hypersensitivity after HP eradication therapy: five patients reported an immediate and 23 patients a delayed reaction (Fig. 1). In addition to, clarithromycin 22 patients had received amoxicillin and six metronidazole. Types of PPI included pantoprazol in 17, omeprazol in nine and esomeprazol in two cases, respectively. By challenge testing, clarithromycin allergy could be excluded in 23 patients whereas one patient developed a macular-papular exanthema during the challenge test (Table 2, Fig. 4). Four patients refused challenge tests with clarithromycin. All skin and challenge tests with metronidazole and PPIs were negative. However, in eight patients amoxicillin allergy was identified as cause for the exanthema by skin testing (Fig. 4).
Steps and results of the allergological work-up in a subset of 28 patients with suspected hypersensitivity symptoms in temporal relation to HP-eradication treatment with clarithromycin. neg.; negative result. pos.; positive result. BAT; basophil activation test. LTT; lymphocyte transformation test.
Macrolides are among the safest antibiotics available for the treatment of bacterial infections. In epidemiological studies urticaria and angio-oedema have been associated with macrolide treatment, whereas delayed skin reactions have been less frequently reported.19,20 Importantly, in daily practice and especially in drug reaction reports, the diagnosis of macrolide hypersensitivity is only based upon history without further diagnostic evaluation.9 However, our study shows that history alone is a vague and unreliable indicator of true macrolide hypersensitivity. Oral challenge tests rule out macrolide hypersensitivity in approximately 90% of patients who had previously been labelled as such. Unfortunately, according to the literature and in our own experience results of macrolide skin or in vitro testing often yield negative results.3 Therefore oral challenge tests seem to be the only way to definitely diagnose or exclude macrolide hypersensitivity.
Prick and intradermal skin testing have been successfully applied in the assessment of IgE-mediated (immediate) immunological drug reactions such as penicillin.12,15 In our study immediate readings of prick and intradermal macrolide tests were all negative – by challenge testing we also did not identify a patient with definite immediate macrolide hypersensitivity. Based on published data the significance of macrolide skin testing for patients with a history of immediate hypersensitivity symptoms remains undetermined.4
Combined prick, intradermal, and patch testing with late readings has been recognised as a reliable tool for diagnosing delayed hypersensitivity with occurrence of delayed reactions at the skin test site, particularly for aminopenicillins.14 In our 72 patients with a history of a delayed skin reaction only one skin test with roxithromycin was positive. In our series, four patients developed exanthema after macrolide challenge testing despite previously negative skin tests. Such negative macrolide skin tests in cases of confirmed delayed hypersensitivity have been previously reported.4,19 One cause for false-negative skin tests may be the fact that the culprit compound of the hypersensitivity reaction is not the parent drug itself, but a metabolite. Methodical limitations of the described skin test procedure, e.g. concentration, vehicle and duration of occlusive patch exposure, may also explain false-negative skin test results.
Oral challenge tests are considered to be the gold standard to establish or exclude drug hypersensitivity.18 Before performing a drug challenge test an assessment of the risk-benefit relationship has to be done and careful surveillance of patients is mandatory. In our series, four patients with suspected delayed hypersensitivity developed late skin reactions between 6 and 20hours after the last dose. A negative result indicates that the patient is not sensitive at the time of the challenge test.21 However, false-negative results can occur due to missing co-factors such as viral infection or exercise or due to tolerance induction by the challenge procedure.18,22 Theoretically, it is also possible that drug challenge tests lead to sensitisation, however this has not been shown to be relevant for e.g. penicillins.23 In our challenge tests we observed delayed-type skin reactions in four patients (three for exanthema and one fixed drug eruption) despite negative skin and laboratory testing. In contrast, in patients with immediate reactions all challenge tests were constantly well tolerated confirming negative skin and laboratory test results. Therefore, compared to immediate-type reactions the negative predictive value of combined skin and laboratory testing in delayed reactions seems to be lower.
In one patient with anaphylaxis grade 3 we measured raised baseline serum tryptase levels (obtained six weeks after resolution of the clinical signs) reflecting an elevated mast cell burden as symptom of systemic mastocytosis. This constitutes a substantial risk factor for severe/hypotensive anaphylaxis triggered by the infectious disease and histamine-liberating drugs given concomitantly with the macrolide.17,24
Laboratory tests with macrolides as further diagnostic methods such as the lymphocyte-transformation-test and the basophil activation test yielded constantly negative results. However, these tests have yet to be standardised, and results depend on methods and laboratory.16,25
Commonly, macrolide-induced delayed skin reactions present as maculo-papular exanthema with or without resemblance to an infectious exanthema.4 However, patients may also develop more specific skin eruptions. Among them, episodes of clinically and histologically verified fixed drug eruption have been most often described. Surprisingly, in one challenged patient we observed a fixed drug eruption despite a history of maculo-papular exanthema. In cases with suspected fixed drug eruption, skin tests should be applied not only on the upper back but also in former lesional skin. In macrolide-induced fixed drug eruption positive skin tests on unaffected skin as well as falsely negative skin tests in former lesional skin have been reported.6,26
Our results of 109 patients with presumed macrolide hypersensitivity tolerating the suspected macrolide demonstrate that symptoms developing in temporal relationship with the intake of a macrolide may have been uncritically attributed to and misinterpreted as signs of hypersensitivity by patients and physicians. How can we explain the high frequency of incorrect diagnosis of macrolide hypersensitivity? Infectious diseases may lead to acute urticaria and angio-oedema. The most frequent reason for acute urticaria appears to be viral infections, especially of the upper respiratory tract, which are usually present a few days before the onset of wheal formation.27 The urticaria is then frequently attributed to the macrolides which were taken concomitantly. Exanthema in temporal relationship to the application of a macrolide may have originated from other causes than the suspected drug, e.g. viral exanthema. Also, other drugs given concomitantly with the macrolide may trigger hypersensitivity symptoms. The subanalysis of the 28 patients after HP treatment identified only one patient with clarithromycin allergy. But additional drug skin and challenge tests performed with the co-prescribed amoxicillin, metronidazole, and PPIs identified amoxicillin allergy in eight cases. Intradermal and patch tests are reliable tools for diagnosis of delayed hypersensitivity to aminopenicillins with a high sensitivity.28 In an earlier study we were able to confirm aminopenicillin allergy in 68 of 71 patients by combined skin testing using intradermal and patch tests with a sensitivity of 95.8%.14 In summary, by skin and challenge tests drug hypersensitivity could be definitely rejected in 15 out of the 28 patients as cause for exanthema or urticaria during HP treatment.
In conclusion, allergological testing in cases of suspected macrolide allergy is of utmost importance either to establish a correct diagnosis or to prevent unjustified macrolide allergy claims. Skin or in vitro testing alone is not sufficient to identify macrolide hypersensitivity and on a risk-benefit assessment oral challenge tests should be offered.
Conflict of interestThe authors have no conflict of interest to declare.