β-lactoglobulin (β-Lg)-sensitized mice model was employed to investigate the correlation between Lactobacillus acidophilus KLDS 1.0738 (Lap KLDS 1.0738) modulating gut microbiota and inducting Toll-like receptors (TLRs) expression.
MethodsThe alterations of mice fecal microbiota were analyzed by 16S rRNA gene sequencing. The serum cytokines production and TLR4/NF-κB mRNA expression in the colon tissues were measured by ELISA kit and quantitative RT-PCR, respectively.
ResultsThe results showed that Lap KLDS 1.0738 pretreatment attenuated β-Lg-induced hypersensitivity, accompanied with a diminished expression of TLR4/NF-κB signaling. Moreover, oral administration of Lap KLDS 1.0738 improved the richness and diversity of fecal microbiota, which was characterized by fewer Proteobacteria phylum and Helicobacteraceae family, and higher Firmicutes phylum and Lachnospiraceae family than allergic group. Notably, TLR4/NF-κB expression was positively correlated with the family of Helicobacteraceae in allergic group, but negatively correlated with the family of Lachnospiraceae, Ruminococcaceae and anti-inflammatory cytokines level. A significant positive correlation was observed between TLR4/NF-κB expression and the production of histamine, total IgE and pro-inflammatory cytokines.
ConclusionsIntake of Lap KLDS 1.0738 can influence the gut bacterial composition, which might result in recognizing TLRs signaling so as to inhibit allergic response.
Recent epidemiologic studies suggested that IgE-mediated cow’s milk allergy (CMA) has a negative impact on infants and young children’s health, which is a serious public health concern.1,2 It has been reported that the intestinal immune barrier dysfunction is partly due to food antigen-induced gastrointestinal microbial dysregulation.3 In general, intestinal flora is considered to modulate the intestinal immune system by stimulating the Toll-like receptors (TLRs) family, and subsequently to result in activating the nuclear factor kinase (NF-κB) pathway, releasing downstream cascade cytokines and differentiating T lymphocyte.4 For example, Shukla et al.5 observed that the intestinal flora disorder in patients with irritable bowel syndrome was positively correlated with up-regulated TLR4 and TLR5 expression and increased IL-6, CXCL-11 and CXCR-3 secretion. Rogier et al.6 indicated that the aberrant intestinal microbiota, induced by IL-1 receptor antagonist deficiency in mice, exacerbated inflammation via TLR4-induced release of pro-inflammatory cytokines and subsequent Th17 differentiation. Therefore, we hypothesized that intestinal microbiota flora disorder induced TLRs activation may act on the pathogenesis of CMA.
Nowadays, probiotics as a novel therapeutic perspective in the prevention of autoimmune and allergic diseases have gained considerable attention.7 Zhang et al.8 highlighted that Lactobacillus rhamnosus GG (LGG) alleviated allergic airway inflammation by regulating the intestinal microbiota, which induced the regulatory T cells (Treg) expression to suppress Th2 responses. Moreover, a clinical human trial also showed that the risk of asthma in infants was reduced by early-life Lactobacillus supplementation through modulating gut microbial diversification and promoting tolerogenic conditions characterized by Treg cell expansion.9 In addition to the general maintaining of the intestinal flora balance, the current studies further illustrated the ability of Lactobacillus to trigger the innate and adaptive immune responses by binding to patterns recognition receptors, such as TLRs signaling molecular.10,11 Kamaladevi et al.12 indicated that Lactobacillus casei elicited the TLR1 mediated p38 mitogen-activated protein kinase (MAPK) pathway to resist Klebsiella pneumoniae infection in Caenorhabditis elegans. Li et al.13 found that Lactobacillus acidophilus significantly inhibited the up-regulation of TLR4, NF-κB and p38 MAPK expression in enterotoxigenic Escherichia coli-infected piglets. However, it is not clear whether intestinal microbiota modulating the TLRs signaling participate in the pathway of Lactobacillus prevent the occurrence of CMA.
We previously demonstrated that heat-killed Lap KLDS 1.0738 induced a protective effect on CMA by improving the Treg/Th17 balance.14 However, further studies are required to analyze its immunomodulatory mechanism. In the present study, the intestinal flora composition and TLRs mRNA expression were evaluated to clarify the anti-allergic pathway of Lap KLDS 1.0738 for its therapeutic effect on CMA. Our findings would provide a new insight for the anti-allergic application of Lap KLDS 1.0738.
Materials and methodsPreparation of lap KLDS 1.0738 and commercial probiotic capsulesThe probiotics strain Lap KLDS 1.0738 obtained from Key Laboratory of Dairy Science (Northeast Agricultural University, Harbin, China) was anaerobically grown overnight at 37℃ in MRS broth (Huankai Microbial, China). The cellular pellets were harvested by centrifugation (5000×g, 10min at 4℃) and washed in sterile saline, and then determined a concentration of 108 or 109CFU/mL and stored frozen at 80℃. The eN-Lac Plus capsule (GenMont Biotec Inc., Taiwan, China) was purchased at retail outlets, which contained 2.0×109CFU/g Lactobacillus (L. parcheese GM-080, L. fermentans GM-090 and L. acidophilus GMNL-185). Before being administered to mice, eN-Lac Plus capsule was suspended in sterilized saline and determined a concentration of 13mg/mL.
Animal modelFemale BALB/C mice at six weeks of age (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were maintained under normal husbandry surroundings with free intake to a milk-free diet. All animal procedures were taken in conformity to the guidelines for animal care and use and approved by the Northeast Agricultural University.
All mice were randomly assigned to five experimental groups (n=6): normal group (N); β-Lg allergic group (β-Lg); commercial probiotics group (CP); low-dose (Lap 1.0738-L); and high-dose (Lap 1.0738-H) Lap KLDS 1.078 group. Except for the normal group, the mice were intraperitoneally injected with 200μL 1mg/mL β-Lg emulsified in incomplete Freund’s adjuvant (Sigma, USA) on days 7, 14, and 21. From day 1 to day 28, the mice in CP group and Lap KLDS 1.078-treated group were oral gavaged 200μL 13mg/mL commercial probiotics (Oral administration with commercial probiotics was calculated to provide approximately 0.13mg/g body weight), 108CFU/mL (Lap 1.0738-L) or 109 CFU/mL (Lap 1.0738-H) Lap KLDS 1.078 three times per week, respectively. At day 28, allergen challenge was performed with all sensitized mice by giving a gavage administration of β-Lg (20mg/mouse) as described previously.15 Two hours later, the blood, fecal and tissue samples from each mouse were immediately collected for further analysis.
Evaluation of allergic symptomsAllergic symptoms were assessed by measuring inflammatory cytokines, histamine and total IgE production in serum. The serum concentrations of histamine and cytokines (IL-1β, IL-6, IL-10, TNF-α, TGF-β) were quantified using the ELISA kits, following the manufacturer’s recommendations (Tiangen, China). The serum total IgE was determined by ELISA according to the previous methods.15
Extraction of RNA, cDNA synthesis, and real-time PCRTotal RNA was isolated from the stored colonic tissues using the Total RNA kit (Tiangen, China) and 2μg of RNA was reverse transcribed to cDNA using FastQuant cDNA (Tiangen, China) as per the manufacturer’s recommendations. Expressions of TLR-4, Myd88, NF-κB were performed by RealMasterMix (SYBR Green) (Tiangen, China) using the ABI 7500 Fast ReaL-Time PCR (Life technologies, USA). PCRs were conducted using the following program: the initial step was 15min at 95°C, then followed by 40 cycles of 10s at 95°C and 30s at 60°C. Primers sequences for target genes were shown in supplementary material. The relative expression of β-actin was used for calculating the levels of target genes. The mRNA level of target gene was determined using the 2−ΔΔCT method.
16S rRNA sequencing and bioinformatical analysisGenomic DNA was extracted from mice feces samples using the E.Z.N.A.®stool DNA Kit (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s recommendations. The amplification of 16S rRNA gene sequences (V3-V4 region) was performed using the TransGen AP221-02: TransStart Fastpfu DNA Polymerase. Purified amplicons were prepared for sequencing on the Illumina Miseq PE300 platform (Illumina Inc., CA, USA). The details of DNA extraction, PCR amplification, quantitative PCR, amplicons purification, sequencing and high-quality sequences criteria are those described in one study.16
The operational taxonomic units (OTUs) were defined as sequences with 97% similarity using UCLUST (v1.2.22) and the Silva (Release119) 16S rRNA database with a 90% confidence threshold. Alpha-diversity indices were used to investigate the bacterial diversity and richness, which were performed using QIIME (version v.1.8). Complex bacterial communities represented by column accumulation diagram were compared at different taxonomic levels, and the column accumulation diagram at different taxonomic levels was drawn by R software (version 2.15.3). Cladogram and linear discriminant analysis (LDA) produced from LEfSe analysis were utilized to identify the differentially dominant bacterial taxa at the OTU level.
Statistical analysisData were displayed as the mean±SD. Statistical difference was analyzed by One-way ANOVA test using the SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The Spearman correlation was applied to reveal the relationship between dominant gut microbiota and allergic symptoms with TLR4 pathway mRNA expression. P-value <0.05 represents a significant difference.
ResultsLap KLDS 1.0738 showed significant inhibitory activity on CMAFirst, we investigated the anti-allergic effect of Lap KLDS 1.0738 in the β-Lg-induced mice model (Table 1). Lap KLDS 1.0738 or commercial probiotics treatment exhibited lower histamine and total IgE (P<0.05), accompanied with increased colon length than those of the allergic group (P<0.05), especially the high dosage of Lap KLDS 1.0738 group. Moreover, oral supplementation Lap KLDS 1.0738 or commercial probiotics also down-regulated the levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and up-regulated the levels of anti-inflammatory cytokine (IL-10 and TGF-β) (P<0.05).
Effects of Lap KLDS 1.0738 on allergic symptoms of β-Lg sensitized mice.
| Item | N | β-Lg | CP | Lap 1.0738-L | Lap 1.0738-H |
|---|---|---|---|---|---|
| Colon length (cm) | 8.48±0.44 | 5.74±0.25* | 6.53±0.47*† | 7.23±0.42*† | 7.87±0.32† |
| Histamine (ng/mL) | 9.93±1.06 | 18.98±0.98* | 16.13±0.73*† | 15.30±0.52*† | 12.58±1.32† |
| Total IgE (ng/mL) | 71.15±2.45 | 133.78±3.05* | 100.47±2.42*† | 94.67±1.53*† | 74.55±3.13† |
| IL-6 (pg/mL) | 66.50±10.22 | 120.80±16.08* | 74.46±7.59† | 94.72±13.44*† | 67.19±8.14† |
| IL-1β (pg/mL) | 41.04±11.36 | 114.53±19.15* | 81.89±3.25*† | 63.56±10.22*† | 57.65±7.11† |
| TNF-α (pg/mL) | 320.34±19.73 | 583.55±10.45* | 467.67±19.30*† | 359.54±7.13*† | 316.77±26.94† |
| IL-10 (pg/mL) | 529.31±11.79 | 387.52±16.31* | 568.21±2.90 *† | 620.04±13.65*† | 627.11±28.09*† |
| TGF-β (pg/mL) | 96.72±6.10 | 79.50±8.00* | 115.83±9.81*† | 137.43±5.21*† | 148.81±8.81*† |
Normal group (N), β-lactoglobulin group (β-Lg), commercial probiotic group (CP), low dose of Lap KLDS 1.0738 group (Lap 1.0738-L), high dose of Lap KLDS 1.0738 group (Lap 1.0738-H). The results are means±SD (n=3). *P<0.05 versus N group. †P<0.05 versus β-Lg group.
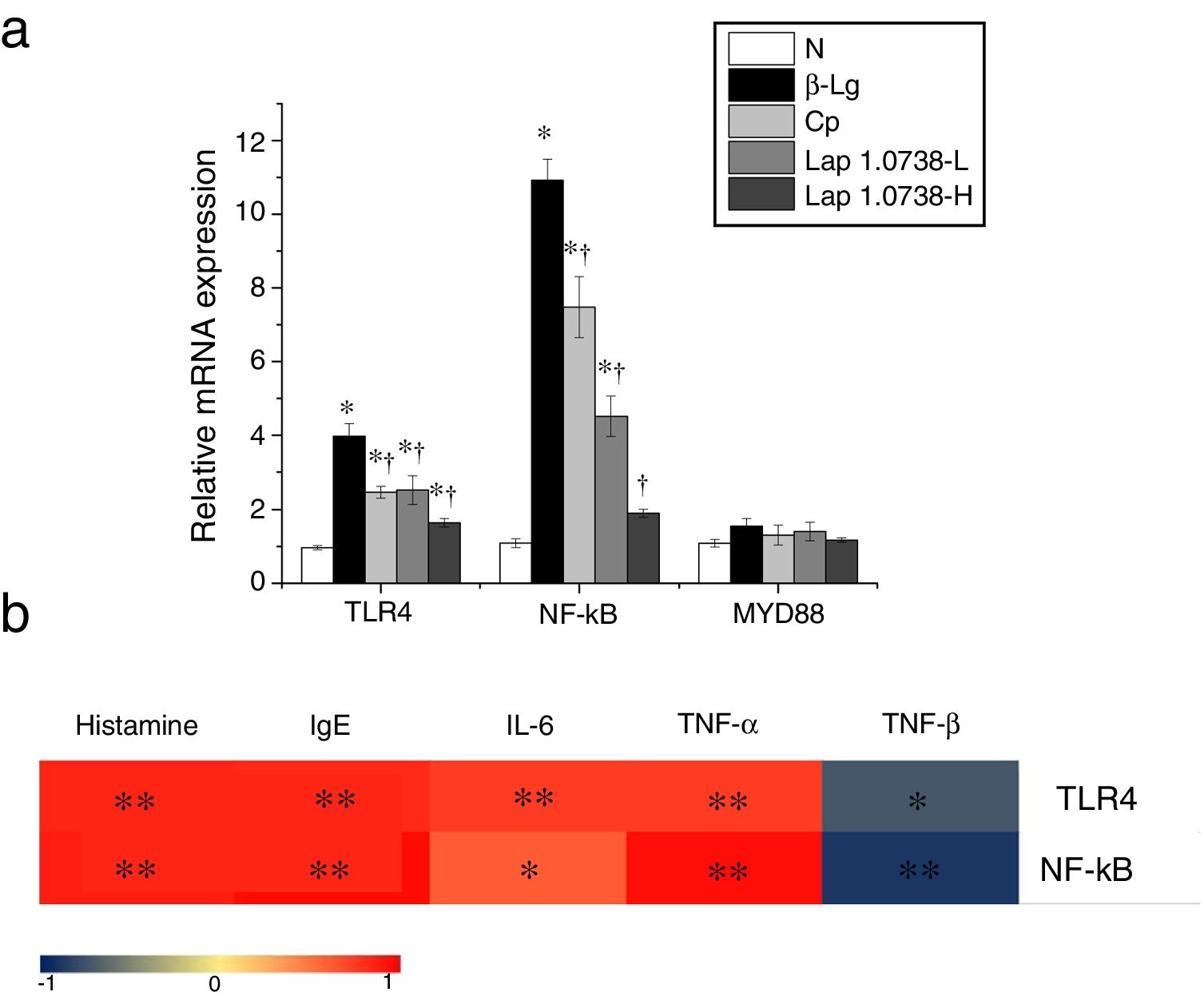
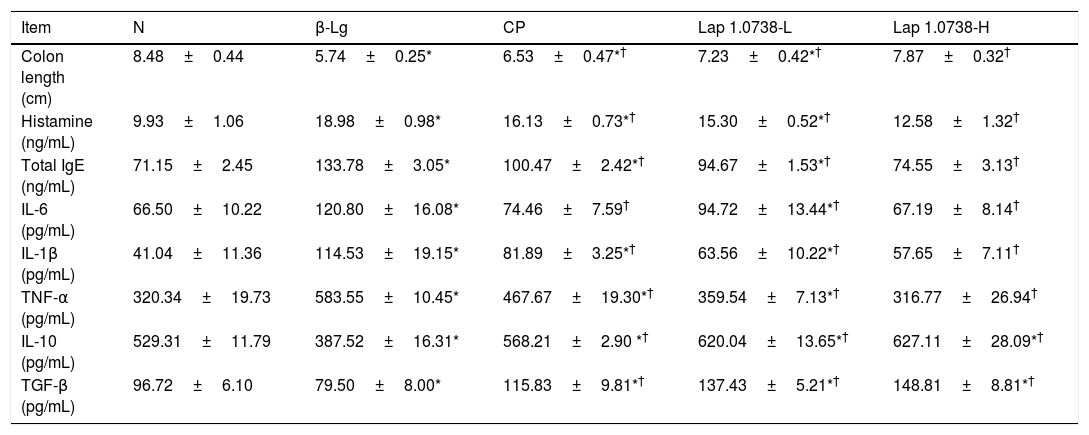
Further data suggested that bovine β-Lg challenge led to aggravation of the expressions of TLR-4 and NF-κB compared with the control (Fig. 1a). In contrast, allergic mice treated with Lap KLDS 1.0738 dose-dependently decreased the TLR4 and NF-κB expression (P<0.05). The Myd88 mRNA expression was not statistically significant in all the experimental treated groups (P>0.05). The trend of commercial probiotics treatment suppressed TLR4 signaling similar to that of Lap KLDS 1.0738-treated groups. Notably, the expressions of TLR4/NF-κB were positively correlated with the production of histamines, total IgE, IL-6 and TNF-α (P<0.05), but were negatively correlated with the secretion of anti-inflammatory cytokines TGF-β (Fig. 1b).
The expression of TLR4/NF-κB signaling (a) and the correlation between the TLR4/NF-κB expression and allergic symptoms (b). The correlation of the expression of TLR4/NF-κB signaling pathway in colonic tissues and the level of histamine, total IgE, IL-6, TNF-α, TGF-β in serum of allergic group compared to treatment groups. Normal group (N), β-lactoglobulin group (β-Lg), commercial probiotic group (CP), low dose of Lap KLDS 1.0738 group (Lap 1.0738-L), high dose of Lap KLDS 1.0738 group (Lap 1.0738-H). *P<0.05 versus N group. †P<0.05 versus β-Lg group. *P<0.05, **P<0.01.
Next, we investigated the effect of probiotics on the fecal microbiome by 16S rRNA gene sequencing. The sequence datasets consisting of 313,132 high-quality classifiable 16SrRNA gene from 15 fecal samples were obtained and an average of 253 OTUs per group were identified. The detailed diversity estimates of fecal microbiota are listed in Table 2. Compared with the allergic group, both of microbial species richness (Chao1) and diversity (Shannon) were increased in Lap KLDS 1.0738-treated groups (P<0.05). The difference in the Shannon and Chao1 index between the high-dose Lap KLDS 1.0738 group and the control was not significant (P>0.05). It was worth noting that the commercial probiotics group had lower a Shannon and Chao1 index than those of the allergic group (P<0.05).
Illumina sequencing data summary.
| Group | Sequences produced | OTU numbers | Shannon | Chao1 |
|---|---|---|---|---|
| N | 21982±6141.327 | 260.333±7.023 | 6.073±0.084 | 261.731±1.086 |
| β-Lg | 11409.5±54.447* | 240.667±3.511* | 5.391±0.017* | 269.177±4.858 |
| CP | 21318±3470.626† | 199.333±7.234*† | 4.660±0.034 *† | 213.133±5.146*† |
| Lap 1.0738-L | 24990.667±4455.059† | 290.333±3.786 *† | 5.517±0.430* | 289.467±3.576*† |
| Lap 1.0738-H | 24677±4370.144† | 272.333±7.506 † | 5.682±0.220 | 276.552±18.468 |
Normal group (N), β-lactoglobulin group (β-Lg), commercial probiotic group (CP), low dose of Lap KLDS 1.0738 group (Lap 1.0738-L), high dose of Lap KLDS 1.0738 group (Lap 1.0738-H). The results are means±SD (n=3). *P<0.05 versus N group. †P<0.05 versus β-Lg group.
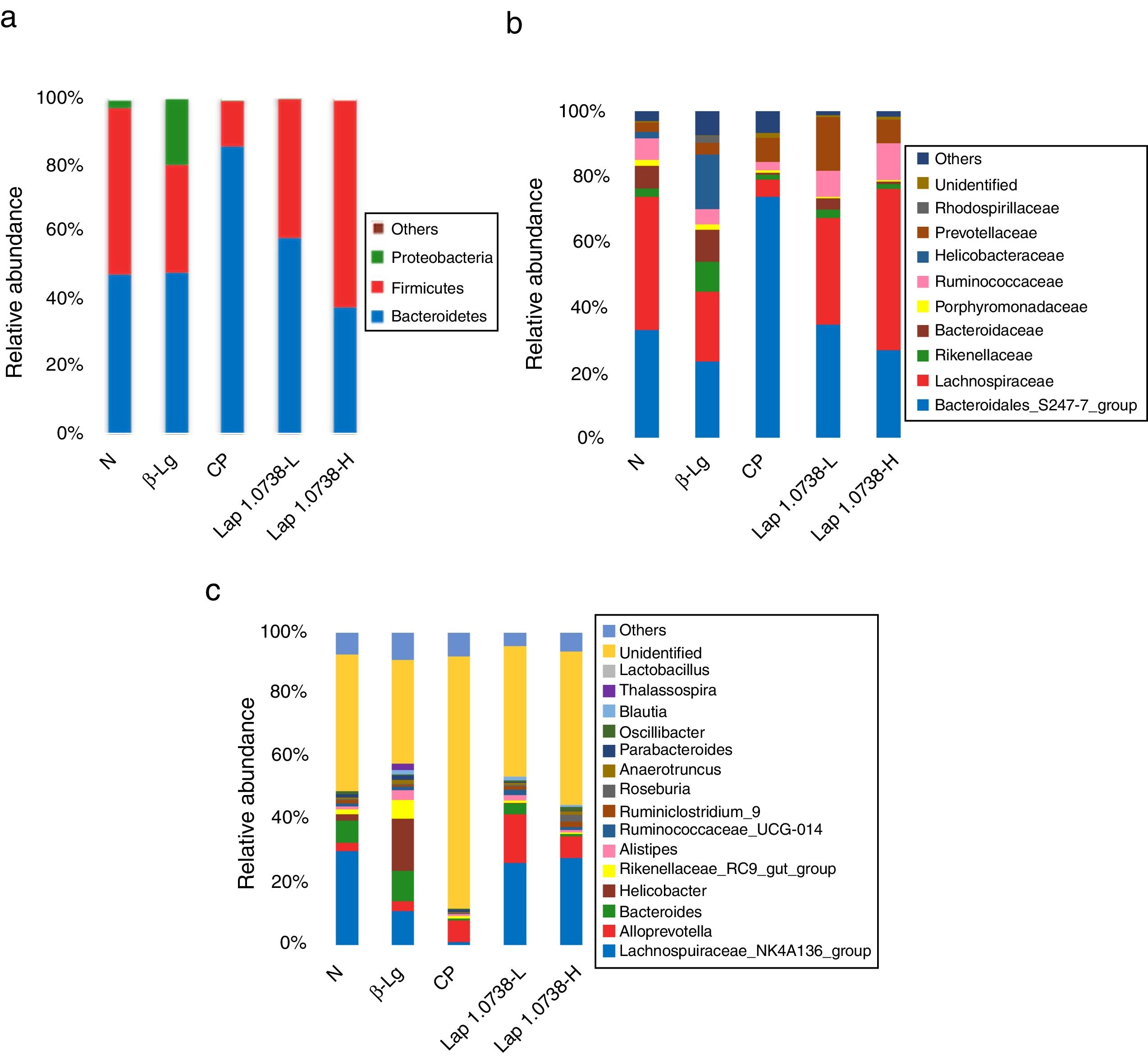
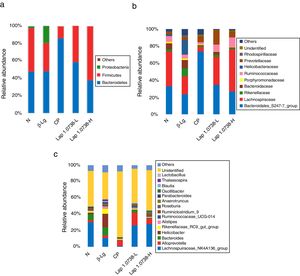
To further explore the microbial species and their relative abundance, the microbiota compositions were analyzed at the phylum, family, and genus levels, respectively (Fig. 2). At the phylum level, it was found that those administered with Lap KLDS 1.0738 reversed the alterations of fecal microbiota induced by β-lg allergen, including prompting the Firmicutes growth (41.62% and 61.97%) and inhibiting the overgrowth of Proteobacteria (0.19% and 0.16%) (P<0.05). Interestingly, the ratio of Bacteroidetes (85.54%) in the commercial probiotics group was significantly higher than the other groups (P<0.05). At the family level, Lap KLDS 1.0738 treatment clearly exhibited higher abundance of Lachnospiraceae (32.63% and 49.25% vs. 21.41%), Bacteroidales_S24-7 (34.72% and 26.94% vs. 23.41%) and Ruminococcaceae (7.96% and 11.28% vs. 4.72%), but a lower proportion of Bacteroidaceae (3.54% and 0.81% vs. 9.86%), Rikenellaceae (2.52% and 1.59% vs. 9.15%) and Helicobacteraceae (0.02% and 0% vs. 16.65%) than those in the allergic group (P<0.05). Although intake of commercial probiotics remarkably decreased Helicobacteraceae (0.01%), Bacteroidaceae (0.63%) and Rikenellaceae (1.52%) levels (P<0.05), but failed to improve Lachnospiraceae (5.27%) and Ruminococcaceae (2.43%) levels. Consumption of Lap KLDS 1.0738 consistently reversed the expansions of Helicobacter, Bacteroides and Rikenellaceae_RC9_gut_Group, but up-regulated the Lachnospiraceae_NK4A136_group, Ruminococcaceae_UCG-014 and Ruminiclostridium_9 at the genus level.
Comparisons of fecal bacterial communities at phylum (a), family (b), and genus (c) levels in all groups. Normal group (N); β-lactoglobulin group (β-Lg); commercial probiotic group (CP); low dose of Lap KLDS 1.0738 group (Lap 1.0738-L); high dose of Lap KLDS 1.0738 group (Lap 1.0738-H).
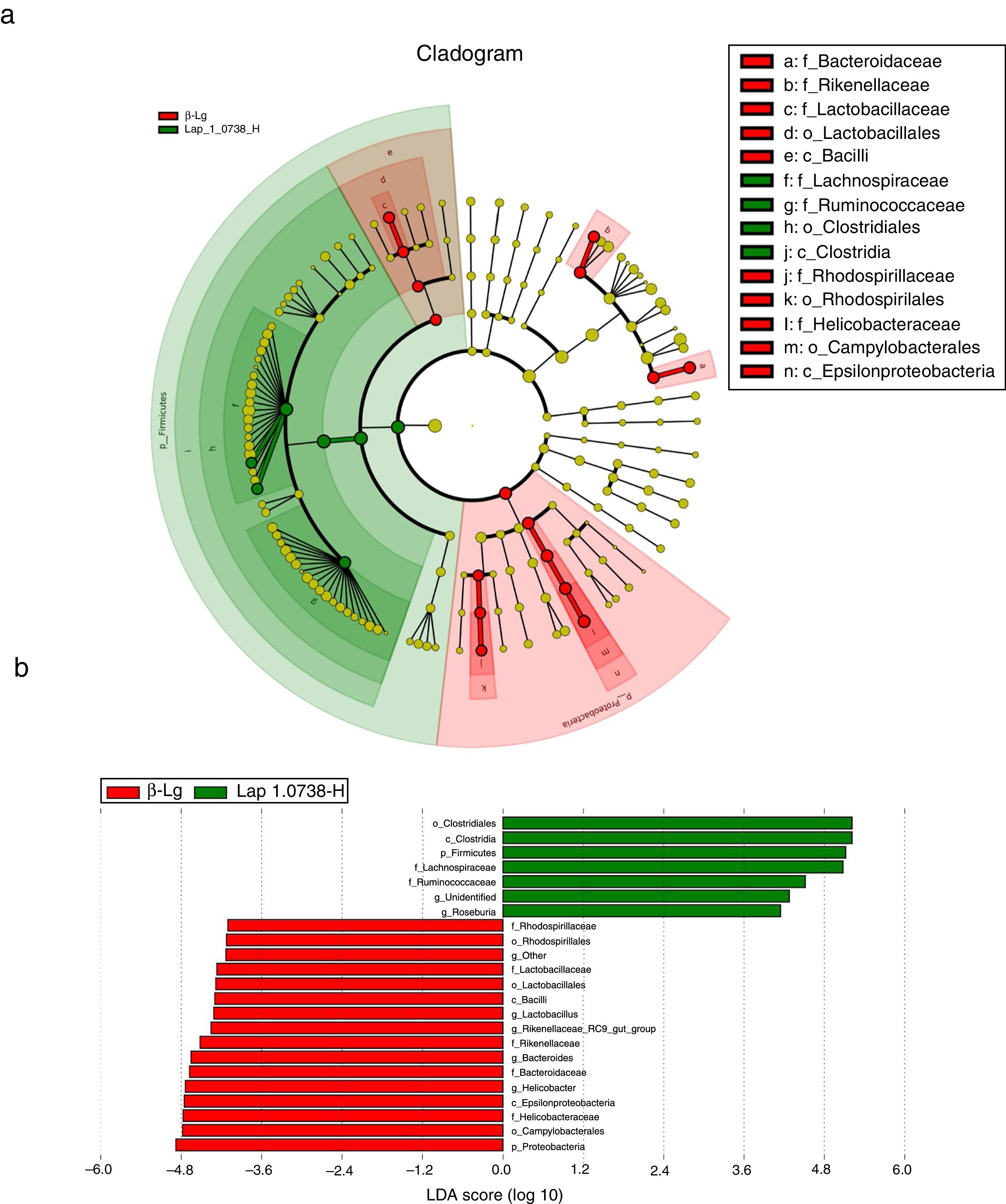
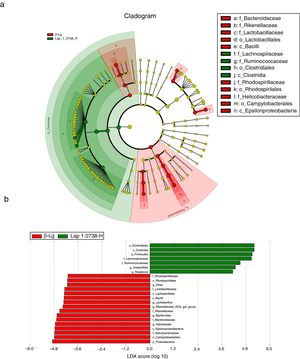
Subsequently, LEfSe analysis was performed to investigate the biomarker species of feces microbiota between the allergic group and the high-dose Lap KLDS 1.0738 group (Fig. 3). The cladogram and linear discriminant analysis scores (LDA, values >4) exhibited 22 dominant taxa (from phylum to genus level), including 15 dominant taxa in allergic group and seven dominant taxa in high-dose Lap KLDS 1.0738 group. The family of Lachnospiraceae and Ruminococcaceae, which belong to Firmicutes phylum (Clostridia class, Clostridiales order), were mainly taxa enriched in the high-dose Lap KLDS 1.0738 group, while the family of Helicobacteraceae belonging to Proteobacteria phylum (Epsilonproteobacteria class, Campylobacterales order) and family of Bacteroidaceae and Rikenellaceae belonging to Bacteroidetes phylum were mainly taxa enriched in the allergic group. Similarly to the family level, allergic mice displayed a significant increase in Helicobacter and Bacteroides at the genus level.
Cladogram (a) and linear discriminant analysis (LDA) (b) produced from LEfSe analysis. LEfSe identifies the most differentially abundant bacterial taxa among groups. Only taxa meeting an LDA significant threshold of >4 are shown. Normal group (N); β-lactoglobulin group (β-Lg); commercial probiotic group (CP); low dose of Lap KLDS 1.0738 group (Lap 1.0738-L); high dose of Lap KLDS 1.0738 group (Lap 1.0738-H).
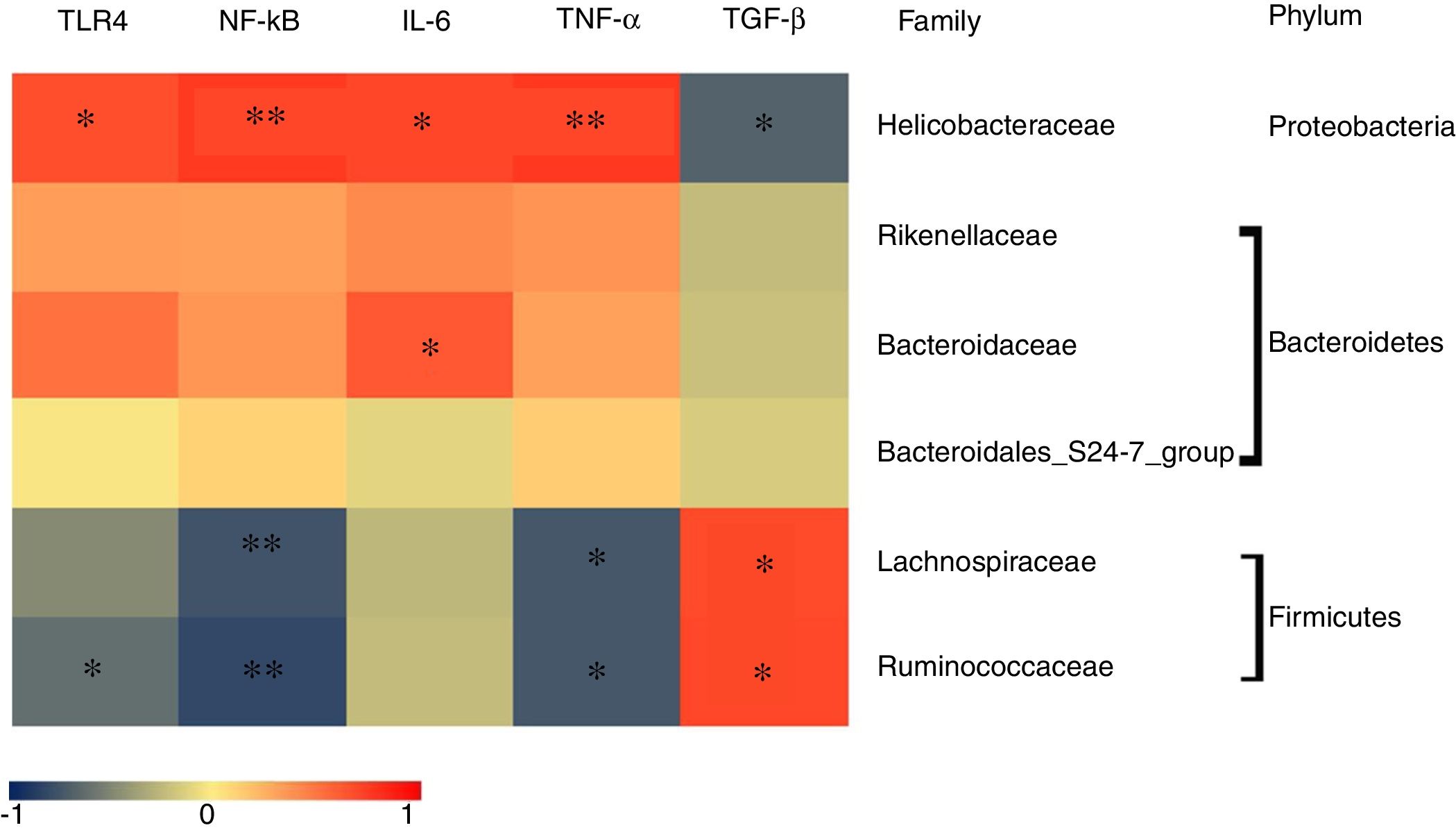
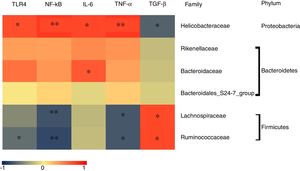
To determine which specific bacteria modulated the colonic mucosal TLRs and thereby attenuated allergic symptoms, the Spearman correlation was performed to compare the dominant bacteria at the family level with TLR4 expression and inflammatory markers. Fig. 4 shows that the abundance of Rikenellaceae, Helicobacteraceae, and Bacteroidaceae were positively correlated with the expression of TRL4 and TRL4-linked NF-κB, especially the Helicobacteraceae family belonging to Proteobacteria phylum (P<0.05). Moreover, a significant positive correlation was observed between the family of Helicobacteraceae and Bacteroidaceae and pro-inflammatory cytokines IL-6 (P<0.05). On the other hand, the abundance of Lachnospiraceae and Ruminococcaceae were significantly negatively related to the expressions of TLR4/NF-κB and the production of pro-inflammatory cytokines TNF-α (P<0.05), but were positively correlated with the anti-inflammatory cytokines TGF-β level (P<0.05). There was no significant relationship between Bacteroidales_S24-7 and TLR4 pathway (P>0.05).
DiscussionNumerous studies have very recently pointed out that the interaction among probiotics and gut microbiota ecology is a critical determinant in maintaining immune system maturation and tolerance acquisition.17,18 In this study, oral Lap KLDS 1.0738 administration displayed inhibitory effects on β-Lg-induced inflammation in an allergic mice model. Furthermore, we observed that the alterations of the gut microbiota mediated by probiotics were associated with reducing the risk of allergic sensitization.
First, Lap KLDS 1.0738-treated restored enteric microbial richness and diversity, which were characterized by lower numbers of Proteobacteria phylum and higher numbers of Firmicutes phylum than the allergic group. The enormous growth of Proteobacteria phylum was considered to be a potential diagnostic signature for gut microbial dysbiosis, which has been observed in various inflammatory disease states.19 For instance, one study of fecal microbiota in patients with IBD exhibited higher proportion of Proteobacteria (particularly Enterobacteriaceae) and the lower level of Firmicutes than those of the healthy controls.20 Similar animal experiments demonstrated that, after probiotics LGG therapy, the changes in phylum-level shift from Firmicutes to Proteobacteria caused by homotypic virulent human rotavirus in pig were effectively prevented and managed.21
Second, the observed differences in family and genus levels revealed that the CMA mice gut microbiota comprised predominantly by the family of Helicobacteraceae, belonging to Proteobacteria phylum, and the family of Bacteroidaceae and Rikenellaceae, belonging to the Bacteroidetes phylum. Similar to family-level analysis, studies at genus level also showed significant enrichment in β-lg-sensitized mice samples of Bacteroides and Helicobacter. The gram-negative Helicobacteraceae was considered a determinant pathogenic role in the intestinal immune system, and has been detected in several enteritis and infection disease models.22,23 Although members of the Bacteroidaceae family represented a potential anti-inflammatory effect in host health,24 the specific taxa exacerbated enteric infection when the gut microbiota was imbalanced. For instance, Curtis et al.25 reported that the increase of Bacteroides could alter intestinal permeability, lead to excessive antigen transfer and hence contribute to perpetuating the inflammatory reaction. Notably, a recent clinical trial reported that CMA children maintained a greater average number of Bacteroides than non-allergic children.26 The Rikenellaceae family was found more often in the intestinal microbiota of disease models than in healthy controls, while its potential risk for inflammation and allergy development remained uncertain. Those observations demonstrated that the damage of the gut immunologic barrier modified the abundance of resident bacteria, leading to exacerbation of the inflammation in β-Lg allergic mice.
Conversely, supplementation with Lap KLDS 1.0738 not only reversed the expansion of potentially pathogenic bacteria, but also remarkably up-regulated the proportion of Lachnospiraceae and Ruminococcaceae (phylum Firmicutes, class Clostridia), as well as Bacteroidales_S24-7 (phylum Bacteroidetes). The family of Lachnospiraceae and Ruminococcaceae are prevalent in the digestive tract of mammals, and have a protective effect on the intestinal immune system because of their ability to produce short-chain fatty acids, especially butyric acid.27,28 Early research focused on their functions link butyric acid to suppress colon cancer and obesity levels.29 Currently, Wang et al.30 found that treatment of colitis mice with B.bifidum increased the abundance of Lachnospiracea-bacterium, which resulted in regulating oxidative stress and inflammatory mediators. Sagheddu et al.31 demonstrated the beneficial role of Ruminococcaceae in promoting protein synthesis and preventing infant malnutrition. Interestingly, consumption of commercial probiotics statistically increased the relative abundance of genus Bacteroidales_S24-7, which was consistent with the increase in the Bacteroidetes phylum. New insights indicated that the presence of Bacteroidales_S24-7 induced accumulation of bacterial extracellular DNA to down-regulate TNF-α, IL-6 levels and up-regulate IL-10. These results were supported by Zhao et al.32 and Qi et al.,33 who demonstrated that the ability of Bacteroidales_S24-7 to maintain immune homeostasis was closely accompanied with inhibiting inflammatory cytokines, including IL-6. Therefore, the regulatory function of Bacteroidales_S24-7 in β-Lg allergy remains in need of further validation.
In general, pathogenic bacteria activate TLR4, resulting in aggravating the inflammatory response in the intestine by influencing downstream NF-κB pathway and cytokines release.34 In this study, the up-regulated TLR4 expression and increased serum IL-6, TNF-α levels were observed in β-lg-induced mice. Moreover, Helicobacteraceae, Bacteroidaceae and Rikenellaceae were found to be involved in the β-lg allergy’s pathogenesis and positively related to TRL4/NF-κB pathway and IL-6. In agreement with our studies, Loganathan et al.35 showed that genetic polymorphisms in TLR4 and TLR9 were associated with chronic Helicobacter pylori-related infection. While dysbiotic Bacteroides species were accompanied by defective innate immunity and ineffective regulatory T cells, which were considered as important factors of IBD pathogenesis. Kim et al.36 indicated that TLR4 was required for Bacteroides fragilis induced NF-κB activation and proinflammatory IL-8 release. In contrast, after the treatment of allergic mice with Lap KLDS 1.0738, TLR4 overexpression and inflammatory cytokines production both were declined, with increasing tendencies in the number of protective gut commensal strains. It is worth noting that increased Lachnospiraceae and Ruminococcaceae in Lap KLDS 1.0738-treated group was positively associated with TGF-β, but a negative association was recognized with TLR4, NF-κB, and TNF-α. In agreement with our studies, the genera Faecalibacterium and Ruminococcus belonging to the family Ruminococcaceae were reported to be inversely correlated with TLR4 expression in Chinese patients with functional gastrointestinal disorders.37 Similarly, the severity of colon inflammation in Nlrp12−/−mice was attenuated by promoting beneficial Lachnospiraceae strains, accompanied by suppressing excessive immune signaling, such as NF-κB, ERK and STAT3.38 Moreover, we found the correlation between MyD88 and dominant gut microbiota showed no significant difference in all groups. Though MyD88 as an adapter molecule was commonly responsible for the TLR-induced inflammatory cytokines, MyD88-independent pathways could be triggered by TLRs ligands to activate NF-κB pathway.39,40 Our previous studies also provided evidence that L. acildophilus peptidoglycan treatment had no effect on Myd88 mRNA level in β-Lg allergic mice.41 Therefore, we speculated that Lap 1.0738’s might protect against β-Lg-induced hypersensitivity through MyD88-independent signaling.
Moreover, some studies found that the TLRs activation plays an important role in the development of allergies.42 The antigen-specific inflammatory markers disorders were mediated by TLR4 and NF-κB signaling mechanism in gliadin and gluten sensitized mice.43 Complete Freund’s adjuvant-induced arthritis mice exhibited higher relative expression of TLR4 signaling, accompanied with increased inflammatory biomarkers (IL-6, IL-1β and TNF-α) levels.44 Our results showed that TLR4/NF-κB signaling was positively associated with the level of histamines, total IgE, IL-6 and TNF-α, but negatively correlated with the production of anti-inflammatory cytokines TGF-β. These correlations could explain that the aberrant intestinal microbiota may be related to TLR signaling overexpression that aggravates immunodysregulation.
ConclusionIn summary, we speculated that the increased number of opportunistic bacterial pathogens might cause TLR4/NF-κB pathway activation, whereas correcting the dysbiosis and altering gut microbiota by administration of Lap KLDS 1.0738 may be contributing to weakening TLR4 transmit signals, which in turn inhibited β-Lg-induced hypersensitivity. Our study potentially provides an important link between Lap KLDS 1.0738 intervention and TLR4 signaling expression through gut microbiota modification in alleviating the β-Lg-induced inflammatory response. In addition, the consumption of Lap KLDS 1.0738 against β-Lg-induced allergy needs to be investigated in further clinical studies.
Conflict of interestThe authors declare that they have no conflict of interest.
This work was supported by the National key R&D Program of China [grant numbers 2017YFD0400304]; the National key R&D Program of China [grant numbers 2018YFD0502404]; and the Heilongjiang province technology fund project [grant numbers C2018022].