Examine the prevalence of asthma and associated predictive factors in a group of 468 students.
Patients and methodsA descriptive, cross-sectional observational study in a randomly selected population of 468 children aged 10–12, in the city of Zaragoza. We used the ISAAC questionnaire on asthma completed by children under supervision of the investigators. We assessed the genetic risk (family history of asthma) and environmental risks. The risk for atopy was assessed by the presence of positive skin prick tests.
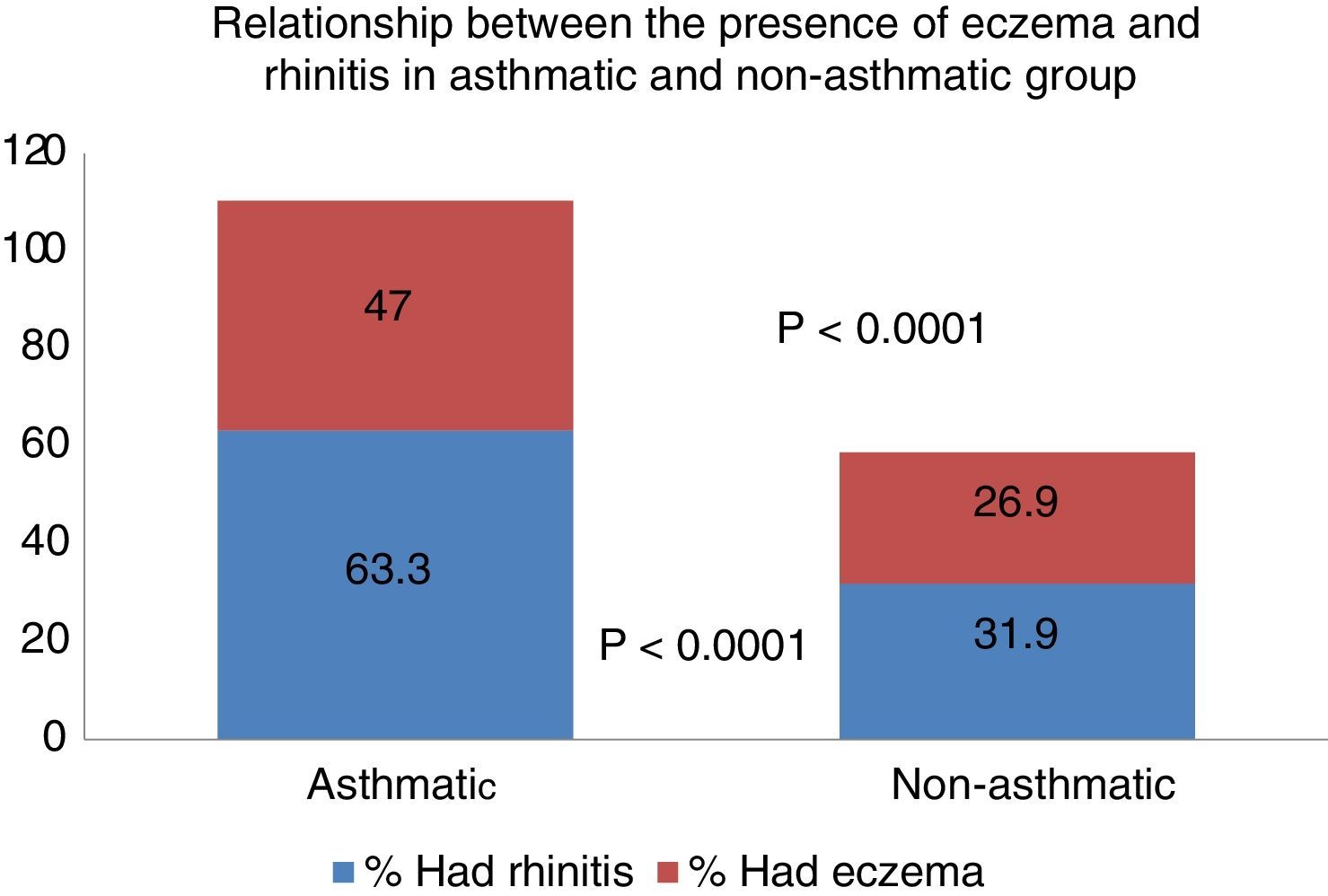
Results25.3% of the children had symptoms consistent with asthma in the city of Zaragoza. Among them 33.1% reported a history of asthma in close relatives (OR=1.78, p<0.001). The history of hospitalisations for lower respiratory tract illness was strongly associated with the presence of asthma (OR=6.72, p<0.0001). Positive skin tests to Alternaria (OR=2.00, p<0.0001) and grass pollen (OR=1.76, p<0.001) were predictors of asthma. 63.6% of asthmatic children had presented clinical rhinitis in the previous 12 months, compared with 32% of non-asthmatics, and this difference was statistically significant (OR=3.89, p<0.0001). 47% of asthmatics presented with or previously had eczema, whereas only 26.9% of non-asthmatics presented with or previously had these types of lesions (p<0.0001).
ConclusionThe following are predictors of asthma: History of hospital admissions for lower respiratory tract illness, presence of rhinitis and/or eczema, positive prick test for certain aeroallergens, especially Alternaria and grass pollen, and family history of asthma.
Asthma is a multifactorial disease with a high prevalence in childhood. It involves a significant degree of morbidity, loss of quality of life for the child and parents and great cost, direct and indirect, to the family and Health Administration.1,2 In Spain, following the epidemiological questionnaire of the International Study of Asthma and Allergy in Children (ISAAC),3 the prevalence of childhood asthma was studied in Huesca,4 Castellón5 and all participating areas.6 The later study included 42,417 Spanish children in phase I (1994–1995), and 42,813 in phase III (2002–2003). The prevalence of asthma in children aged 6–7 years, reported by parents, was 7% and 10.7% for boys in phases I and III and 5.3% and 8.2% for girls in phases I and III, respectively. The self-reported prevalence of asthma in adolescents aged 13–14 years was 9% and 9.3% in boys, in phases I and III, and 9.6% and 9.2% in girls in phases I and III, respectively. However, the results on the prevalence of asthma in many Western countries, even within the same country, are not always comparable, given the geographical, cultural and socioeconomic differences that produce wide variations in the frequency of this disease, nationally and internationally. These differences7 would probably be due to environmental factors (level of hygiene, allergenic pressure, infections, overuse of antibiotics and other unknown factors).
Following the same ISAAC epidemiological questionnaire, the aim of this study was to estimate the prevalence of asthma and its associated predictors in a school population of the city of Zaragoza, Spain. It is named the LAPIZ study (Estudio epidemiLógico de Asma en Población Infantil de Zaragoza [Epidemiological Study of Asthma in the Juvenile Population of Zaragoza]).
Patients and methodsThe design was a descriptive, cross-sectional, observational study of a representative population of 468 children aged 10–12 years in the city of Zaragoza. The study was approved by the local health authority (Instituto Aragonés de Ciencias de la Salud – IACS) and its Ethics Committee. Children of this age were chosen for their comprehension and ability to self-complete the questionnaire, which is not possible for younger children, and for being the oldest group that had afternoon classes once our business hours were over. In the random sampling 14 schools were included, public or semi-private, and one private.
The required sample size calculation was 457 children, making a rough estimate of the population group of 10–12 years of 11,219 people (based on official figures from the current census during the design of the study that showed 28,047 children aged 10–14 years), for a confidence level of 95%, accuracy of 2% and prevalence of childhood asthma of 5%. The sample represented different areas of the city and took place during the autumn and winter months of the 2007–2008 school academic year. We excluded spring and summer because hypersensitivity to grass pollen, olive and Chenopodiaceae, which pollinate during those months, is the most relevant in our geographic area. We thus avoided children receiving symptomatic treatment at the time of the study, which could bias the results. The study only included children whose parents had signed the informed consent. Non-participants were sent an anonymous and voluntary survey asking why they had decided not to participate: forgetfulness; being already diagnosed with asthma and/or allergy; the child does not have any health problems; the child did not want to participate; the parents have not received the informed consent to sign; other causes.
At the outset, the children self-completed the validated ISAAC questionnaire8 for asthma and allergy under the investigators’ supervision. We chose this questionnaire for validation due to its great relevance worldwide. Self-completion yielded two groups: those who had no symptoms suggestive of asthma and those who, in the last 12 months, had experienced asthmatic symptoms and/or had been diagnosed with asthma. Risk factors for asthma assessed in the questionnaire9 were genetic risk (family history of asthma) and environmental risk (exposure to tobacco smoke, domestic animals and relevant respiratory illness). The risk for atopy was assessed by testing the presence of immediate hypersensitivity through skin prick test to one of the most significant environmental aeroallergens in our environment (Bial-Arístegui, Bilbao, Spain), including Phleum pratense, Olea europaea, Salsola kali, Parietaria judaica, Platanus acerifolia, Cupressus sempervirens, Dermatophagoides spp., Alternaria alternata and dog and cat dander. Histamine hydrochloride (10mg/mL) and glycerol saline served as positive and negative controls respectively. A skin test was considered positive when its wheal diameter was larger ≥3mm than the negative control.
Statistical analysisSPSS (version 15) was used to conduct the statistical analysis. Quantitative variables were expressed by their mean and standard deviation and qualitative variables as proportions. Relationships between paired variables were analysed using contingency tables and Pearson contrasts χ2 for qualitative variables. In the case of a qualitative and quantitative variable, relationships were analysed by Student t-test for comparison of means. Finally, multivariate logistic regression was used to identify factors predicting asthma.
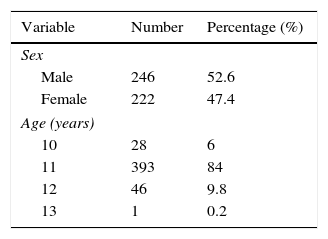
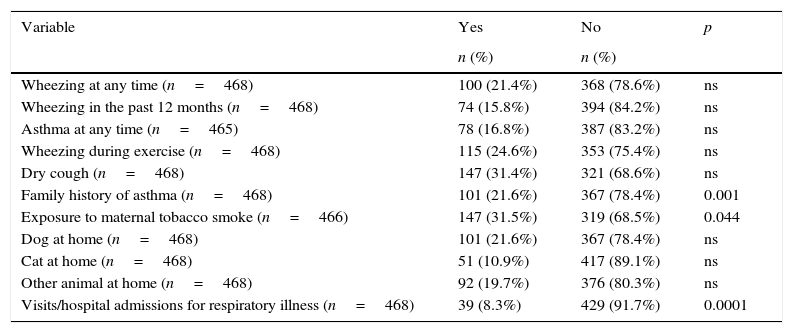
ResultsOf an initial sample of 848 children, 468 participated in our study, representing 55%. The demographic characteristics of non-participants did not differ from the sample: the schools and homes of the children were in the same metropolitan area with similar air pollution and they were studying the same academic year. The anonymous and voluntary survey sent to non-participants was returned by 211 parents (55.53%) and the reasons given were: forgetfulness 6.16% (n=13); being already diagnosed with asthma and/or allergy 12.79% (n=27); the child does not have any health problems 33.17% (n=70); the child did not want to participate 18% (n=38); the parents have not received the informed consent to sign 2.84% (n=6), and other causes 27.01% (n=57). Table 1 shows the general characteristics of the study population. According to the results of the ISAAC questionnaire, 118 of 466 children had symptoms consistent with asthma, those classified as possible asthmatic representing 25.3%. They are children who answered yes to one or both of two questions: “Have you had wheezing or whistling in the chest in the last 12 months?” and, “Have you ever had asthma?” The estimated prevalence of asthma, according to responses to the first question, was 15.8%. An affirmative answer to the second question equates with diagnosed asthma and involves a previous diagnosis by a doctor. In this case the estimated prevalence of asthma was 16.8%. This percentage is similar to that obtained when asked if they had ever presented wheezing or whistling in the chest in the last 12 months. There were no significant differences in gender distribution. Thirty-three point one percent (33.1%) of asthmatic children answered yes to the question, “Is there a history of asthma in the immediate family?”, which is statistically significant (OR=1.78, 95% CI 1.05–3.01, p<0.001). A relevant number of asthmatics are assumed to be exposed routinely to tobacco smoke at home, with fathers more likely to be smokers than mothers (39%), but only maternal smoking was slightly associated with the presence of asthma (p<0.044). The tendency in developed countries to have pets in the homes is confirmed in our series in which we observed that 52% of the total sample of children surveyed had animals at home, the dog being the most common, followed by cat, hamster and parakeet. Within the group of asthmatic children, 52.5% had a pet. However, living with pets showed no statistical significance. Finally, when asked for a prior history of hospitalisations due to lower respiratory illness, 22% of children with asthma responded positively (OR=6.72, 95% CI 3.08–14.70, p<0.0001) (Table 2).
Prevalence of asthma symptoms and associated risk factors.
| Variable | Yes | No | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Wheezing at any time (n=468) | 100 (21.4%) | 368 (78.6%) | ns |
| Wheezing in the past 12 months (n=468) | 74 (15.8%) | 394 (84.2%) | ns |
| Asthma at any time (n=465) | 78 (16.8%) | 387 (83.2%) | ns |
| Wheezing during exercise (n=468) | 115 (24.6%) | 353 (75.4%) | ns |
| Dry cough (n=468) | 147 (31.4%) | 321 (68.6%) | ns |
| Family history of asthma (n=468) | 101 (21.6%) | 367 (78.4%) | 0.001 |
| Exposure to maternal tobacco smoke (n=466) | 147 (31.5%) | 319 (68.5%) | 0.044 |
| Dog at home (n=468) | 101 (21.6%) | 367 (78.4%) | ns |
| Cat at home (n=468) | 51 (10.9%) | 417 (89.1%) | ns |
| Other animal at home (n=468) | 92 (19.7%) | 376 (80.3%) | ns |
| Visits/hospital admissions for respiratory illness (n=468) | 39 (8.3%) | 429 (91.7%) | 0.0001 |
ns, not significant.
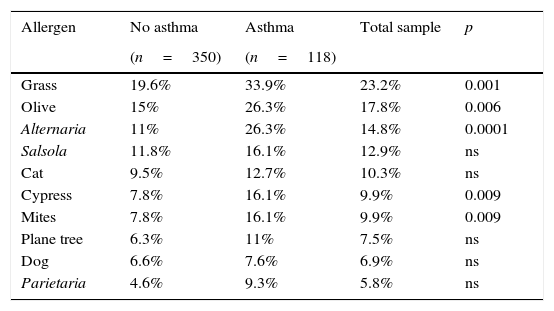
The results of the skin tests in the total sample appear in Table 3. As expected, hypersensitivity to grass pollen was the most frequent (n=108, 23.2%), followed by hypersensitivity to olive pollen (n=83, 17.8%), aerogenic fungus Alternaria (n=69, 14.8%), Salsola (n=60, 12.9%) and cat dander (n=48, 10.3%). Hypersensitivity to mites, plane tree pollen, Parietaria and dog dander was less relevant. Skin tests that were most frequently positive in asthmatic children were the same as in the total sample. Thirty-four percent (34%) showed hypersensitivity to grass pollen and 26% to olive pollen and Alternaria, equally. Skin tests positive for grass, olive, Alternaria, cypress and mites were present in asthmatic children at a rate higher than that observed in non-asthmatics, resulting in statistically significant data (Table 3). Positive skin tests to Alternaria (OR=2.00, 95% CI 1.07–3.75, p<0.0001) and grass pollen (OR=1.76, 95% CI 1.02–3.01, p<0.001) were predictors of asthma. Overall, children with asthma had more frequent positive skin tests to aeroallergens, both in terms of a single test and two or more positive tests (OR=2.08, 95% CI 1.30–3.40, p=0.008).
Skin tests in asthmatics. Relationship between the presence of asthma and positive skin tests.
| Allergen | No asthma | Asthma | Total sample | p |
|---|---|---|---|---|
| (n=350) | (n=118) | |||
| Grass | 19.6% | 33.9% | 23.2% | 0.001 |
| Olive | 15% | 26.3% | 17.8% | 0.006 |
| Alternaria | 11% | 26.3% | 14.8% | 0.0001 |
| Salsola | 11.8% | 16.1% | 12.9% | ns |
| Cat | 9.5% | 12.7% | 10.3% | ns |
| Cypress | 7.8% | 16.1% | 9.9% | 0.009 |
| Mites | 7.8% | 16.1% | 9.9% | 0.009 |
| Plane tree | 6.3% | 11% | 7.5% | ns |
| Dog | 6.6% | 7.6% | 6.9% | ns |
| Parietaria | 4.6% | 9.3% | 5.8% | ns |
ns, not significant.
More than half of the children (52.4%) answered “yes” to the question whether, at some time, they had suffered symptoms of rhinitis, without it coinciding with a cold. When asked specifically whether they had had symptoms of rhinitis in the past year, the number of children who responded affirmatively dropped to 39.7%. To another question, considered especially relevant (Have you ever had hay fever/rhinitis/allergic conjunctivitis?), 17.8% responded affirmatively. Sixty-three point six percent (63.6%) of asthmatic children presented symptoms of rhinitis in the last 12 months, compared to 31.9% of non-asthmatics, and this difference was statistically significant (OR=3.77, 95% CI 2.30–6.18, p<0.0001).
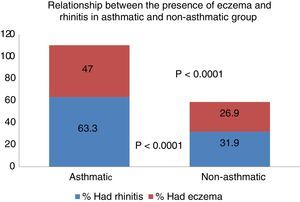
Ninety children (19.2%) of the total sample said they had occasionally suffered an itchy rash compatible with atopic eczema. When asked if it had occurred in the past year, the number decreased to 52 (11.1%). With respect to the relationship between the presence of asthma and eczema, 47% of asthmatics had or had previously presented eczema lesions, whereas only 26.9% of non-asthmatics had or had previously presented these lesions, resulting in a significant difference (p<0.0001) (Fig. 1).
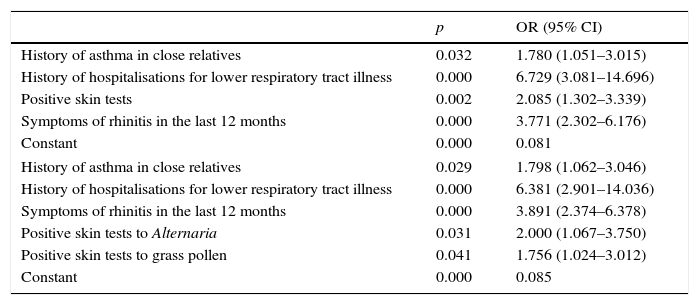
Multivariate logistic regression results are shown in Table 4.
Multivariate logistic regression results.
| p | OR (95% CI) | |
|---|---|---|
| History of asthma in close relatives | 0.032 | 1.780 (1.051–3.015) |
| History of hospitalisations for lower respiratory tract illness | 0.000 | 6.729 (3.081–14.696) |
| Positive skin tests | 0.002 | 2.085 (1.302–3.339) |
| Symptoms of rhinitis in the last 12 months | 0.000 | 3.771 (2.302–6.176) |
| Constant | 0.000 | 0.081 |
| History of asthma in close relatives | 0.029 | 1.798 (1.062–3.046) |
| History of hospitalisations for lower respiratory tract illness | 0.000 | 6.381 (2.901–14.036) |
| Symptoms of rhinitis in the last 12 months | 0.000 | 3.891 (2.374–6.378) |
| Positive skin tests to Alternaria | 0.031 | 2.000 (1.067–3.750) |
| Positive skin tests to grass pollen | 0.041 | 1.756 (1.024–3.012) |
| Constant | 0.000 | 0.085 |
Fifteen point eight percent (15.8%) of the children reported wheezing in the last 12 months. When we assessed children diagnosed with asthma, meaning diagnosed by a doctor, the percentage was similar: 16.8%. The values are higher than would be expected initially, when compared with those obtained by the ISAAC study in Spain by García-Marcos et al.6 but lower than Carvajal-Urueña et al.10 on Spain's northern Atlantic coast. The high prevalence of children with symptoms compatible with asthma in our study is likely due to our including non-asthmatic children within the group of asthmatics. This is one of the limitations of this type of questionnaire. Like the ISAAC study questionnaire, it allows us to make comparisons between different clinical settings and geographic areas, using the same methodology. We must bear in mind, however, that the purpose of these questionnaires is to conduct an epidemiological study and not to formulate a clinical diagnosis. Other limitations of our study are that the cross-sectional design limits the assessment of risk factors, the low participation, the lack of objective measures of asthma, and the absence of some other risk factors for asthma (social class, truck traffic, maternal age, etc.).
In our study there were no significant differences in gender distribution. Arnedo-Pena et al.11 demonstrate that male gender is a risk factor for asthma.
A family history of asthma is very relevant in asthmatic patients.12,13 This is also reflected in our results, in which we see a significant association between asthma symptoms and a family history of asthma; the presence of close relatives with asthma would be a predictor of the disease. Consequently, early diagnosis is desirable in children with a close family history of symptoms suggestive of asthma.
Smoking is the leading cause of preventable disease in the developed world. We know that inhaling tobacco smoke, both active and passive, can cause asthma exacerbations. Some authors even point to tobacco smoke not only to cause exacerbations but also as a direct inducer.14,15 Arnedo-Pena et al.11 demonstrates that the father smoking at home is a risk factor for asthma. Our epidemiological study found that maternal smoking, but not the father's, was significantly associated with the presence of asthma. This can easily be explained: the mother is with the child far more hours than the child's father. Unfortunately, smoking has increased significantly among women in recent decades, becoming a relevant and completely avoidable risk factor. Hence it is important to continue to promote anti-smoking campaigns.
Many epidemiological studies have attempted to assess the possible correlation between pet exposure and the prevalence of asthma and other allergic diseases.16,17 In recent years there have been studies that cast doubt on the influence of exposure to these animals as inducing IgE-mediated sensitisation. For Almqvist et al.18 the coexistence of children with certain pets in the early stages of life would not increase the risk of asthma. Arnedo-Pena et al.11 demonstrates that the presence of a dog at home is a risk factor for asthma. In our series, we found no significant relationship between the presence of asthma and exposure to dog, cat or another pet. Much more striking was the correlation between the presence of asthma and a history of hospitalisations for respiratory illness. For years we have known that infant bronchiolitis may act as a factor inducing asthma.11,19,20 Although the questionnaire does not explore the infectious disease that caused the hospitalisation, presumably in many cases it would be bronchiolitis or another severe respiratory infection.
More than half of the children with asthma showed positive allergy skin tests. The positivity to a single allergen, two allergens, or more than two allergens was significantly higher in asthmatic children than in those who were not. The highest percentage of positive skin tests, both in the total study population as well as in asthmatic children, was to grass pollen, followed by olive and Alternaria, consistent with the geographical and climatic characteristics of the middle Ebro valley.21 Deserving special mention is sensitisation to Alternaria, especially significant in children and adolescents with asthma.22,23 In our series it appears as the third most frequent sensitisation, to the point that 26% of asthmatic children showed positive prick test to this fungus. In many cases this sensitisation is associated with more severe forms of asthma and worse outcomes,24 so that, for some authors,25 sensitisation and exposure to Alternaria alternata is a clear predisposing factor for severe exacerbation in children, adolescents and young adults with asthma.
The current scientific community sees rhinitis and asthma as a single disease26,27 that affects the different levels of the airway. Hence, from a clinical, pathophysiological and epidemiological standpoint, they are closely interrelated.28 In our study, 63.6% of asthmatic children had shown symptoms of rhinitis in the last 12 months, compared to 31.9% of non-asthmatics, representing a significant difference. It is well known that the presence of rhinitis is a risk factor associated with the incidence of asthma,11,29 a fact confirmed in our study. In parallel, 47% of asthmatic children presented or had presented eczema occasionally versus 26.9% of non-asthmatics, an equally significant finding. Therefore, in accordance with the general acceptance,30 the presence of eczema in our children population was associated with the prevalence of asthma.
In conclusion, we believe that, in relation to the questionnaire responses, between 15.8% and 25.3% of children had symptoms consistent with asthma in the city of Zaragoza. The history of hospital admissions for lower respiratory tract illness, the presence of rhinitis and/or eczema, the prick test positive to certain aeroallergens, especially Alternaria, family history of asthma and maternal smoking are risk factors associated with the development of asthma.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingAragonese Foundation for Research Development in Allergology (FADIA).
Conflict of interestNone of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
We thank the nurses of the Allergy Department of the Clinical University Hospital in Zaragoza (Carmen Abadía, Bertha Ariza, Ascensión Bretos, Virginia Roche, Javier Saldaña and Lourdes Sobrino) and the rest of the medical staff (Mar Garcés, María Asunción Domínguez, María Dolores Yécora and Estibalitz Goienetxe).
The Aragonese Foundation for Research Development in Allergology (FADIA) provided funding for this research.