Preschool-aged group is frequently affected by urticaria, and infections are the most frequently documented factors that cause acute urticaria in children. This prospective study was designed to investigate the underlying factors of acute urticaria in under five-year-old children and to describe predictive factors for progression to chronicity or recurrence after the first attack.
Patients and methodsChildren younger than five years of age with acute urticaria were recruited between July 2015 and July 2016. Patients (n=83) were grouped into those below and above two years of age. In order to assess the risk factors for progression to chronicity or recurrence, logistic regression analysis was performed.
ResultsUpper respiratory tract infection was the most common detectable reason for acute urticaria (49.4%). Herpes Simplex Virus type 1 was significantly isolated in the cases with the manifestation of an acute single-episode urticaria (p=0.042). Angioedema and food allergy were predominantly observed under two years old (p=0.001, p=0.006 respectively). A positive relationship was determined between the duration of urticaria and chronicity (r=0.301, p=0.006). The absence of atopic dermatitis (OR: 6.95, 95% CI: 1.35–35.67, p=0.020), negative Herpes virus serology (OR: 4.25, 95% CI: 0.83–21.56, p=0.040), and unknown etiology (OR: 3.30, 95% CI: 1.12–9.71, p=0.030) were the independent risk factors for recurrent urticaria.
ConclusionsPreschool-aged children with acute urticaria should be evaluated for infections at the time of admission. Patients with unknown etiology, negative Herpes virus serology, absence of atopic dermatitis, and long lasting urticaria should be followed up for chronicity and recurrence.
Urticaria is defined as a cutaneous illness characterized by temporary, erythematous and pruritic wheals.1 The accurate diagnosis of underlying cause of an urticarial rash in a pediatric patient is required in order to prevent irrelevant work up for self-limited circumstances and to determine the possibility of recurrence or the progression to chronicity. The contribution of a precise history as a first step to establish the underlying etiology is crucial.2 The determination of causative factors varies between 20% and 90% in different studies.3,4 Infections, followed by food intake, are the most frequently documented factors in children with acute urticaria.4,5
Urticaria is one of the most commonly encountered skin disorders in the pediatric emergency department,6 and causes severe anxiety especially in parents. The identification of the underlying etiology of urticaria might be beneficial for the physician in the management and follow up of the patients, and may reduce the anxiety of parents. The preschool-age group, especially under two years old, is frequently affected by urticaria, and the prevalence of urticaria decreases promptly in school-aged children.7
This prospective study was designed to investigate the underlying factors of acute urticaria (AU) in under five-year-old children and to describe the predictive factors for progression to chronicity or recurrence following the first onset of acute urticaria.
Materials and methodsIn this prospective study, the children younger than five years of age with AU were recruited either from the Pediatric Allergy or the Pediatric Emergency Departments of Hacettepe University Children's Hospital. Patients enrolled into the study between July 2015 and July 2016 and were followed-up until July 2017. The diagnosis of AU was made according to international guidelines.1 Our study was completed in accordance with the ethical standards specified in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Hacettepe University. All parents provided informed consent.
Patient populationThe initial evaluation concerned administration of a survey including demographic characteristics (age, gender, atopic diseases, family history for atopic diseases), symptomatology of AU episode (clinical presentation, duration of urticaria, concomitant angioedema, fever) and possible triggers (recent infections, drug intake, newly introduced foods, insect bites, contact agents, any trigger that parents thought as the etiology). Duration of the urticaria was defined as the period from the beginning to disappearance of the lesions. Unknown etiology was named when parents refused any exposure to environmental triggers and/or clinical or laboratory parameters revealed non-specific findings.
Children were divided into two groups; those below or above two years of age, since patients under two years are often affected by urticaria. All the patients were followed until hives disappeared and were called for control visits three months after the first urticaria attack. Patients were admitted to our department for a second control visit after the first attack of urticaria. At the end of the first and the second years; patients were questioned by telephone, whether they experienced any chronicity or recurrence during the two-year follow-up period.
Definition of urticaria and urticaria activity scoreUrticarial lesions with the duration of less than six weeks named as acute urticaria, and lesion insist longer than six weeks are termed chronic urticaria.8 Recurrent acute urticaria refers to occurrence of hives with a six-week symptom-free interval of urticaria episodes.9 According to the literature, we accepted recurrent urticaria as independent urticaria attacks with six-week lesion-free period after initial acute urticarial lesions, and these attacks were also no longer than six weeks. Urticarial lesions of the patients were grouped as acute, recurrent, and chronic in this study.
Urticaria activity score (UAS) was adapted for AU, and was calculated for all patients. This scoring system was based on the intensity of pruritus and the number of wheals. Scoring was grouped, according to wheal numbers, as: mild (1 point: <20/24h); moderate (2 points: 20–50/24h); and severe (3 points: >50/24h). Additionally, itching intensity was evaluated as: absent (0 point); mild (1 point: no disturbance of sleeping at night but pruritus at daytime); moderate (2 points: moderate pruritus at night); and severe (3 points: disturbance of sleeping at night).10 UAS was calculated as the sum scores of the daily number of wheals and the itch severity.10 UAS was classified as mild (score<16) or moderate/severe (score≥16).10
Laboratory evaluationLaboratory tests performed at enrolment were complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and total IgE. Microbiological examinations included sampling of urine and throat swab for bacterial culture, stool for direct examination and parasites. The most commonly encountered agents in acute urticaria,9 Epstein Barr Virus (EBV) IgM, Cytomegalovirus (CMV) IgM, Mycoplasma IgM, Herpes Simplex Virus (HSV) type 1 and 2 IgM, were studied by Western Blot method in serum samples.
Skin prick testing was done on the child's upper back with a battery of 14 aeroallergens (house dust mites; grass, weed and tree pollens; molds; cat and dog dander; cockroach) with positive (10mg/ml of histamine phosphate) and negative (0.9% sterile saline) controls after resolution of urticarial symptoms and discontinuation of antihistamines. Skin testing with food allergens (cow's milk, egg white, soy, peanut, walnut, hazelnut, wheat, sesame, lentil, chickpea) was performed in infants younger than one year old or in any case with the offending antigen if there was a suspicion of food allergy. Skin test indurations >3mm than that of the negative control were considered positive. In the event of any positivity on SPT and/or specific IgE (Pharmacia Diagnostic AB, Uppsala, Sweden) with culprit food; oral food challenge was performed to confirm food allergy diagnosis. Based on to the patient's history, if the suspected trigger of urticaria was a drug; skin prick and intradermal tests with the culprit drug were performed according to a validated or previously published skin test protocol that was available for the respective drug.11 In cases of negative skin tests or unavailability of the injectable form of the drug, oral provocation test was performed with the culprit drug with increasing doses at appropriate time points.12
Statistical analysisStatistical analyses were performed using SPSS 22 software program (SPSS Inc., Chicago, IL, USA). Variables were investigated using visual and analytic methods to determine whether or not they were normally distributed. Quantitative variables were non-normally distributed and expressed as median [(Interquartile Range (IQR)]. Comparisons of quantitative variables were performed using Mann–Whitney U test. In order to assess the risk factors for progression to chronicity or recurrence, initially univariate logistic regression analysis was performed. Then, using the significant variables (p<0.20), multivariate logistic regression analyses were performed and odds ratios (ORs) with relevant 95% confidence intervals (CIs) were calculated. While investigating the associations between non-normally distributed and/or ordinal variables, the correlation coefficients and their significance were calculated using the Spearman test. A p value <0.05 was considered as statistically significant.
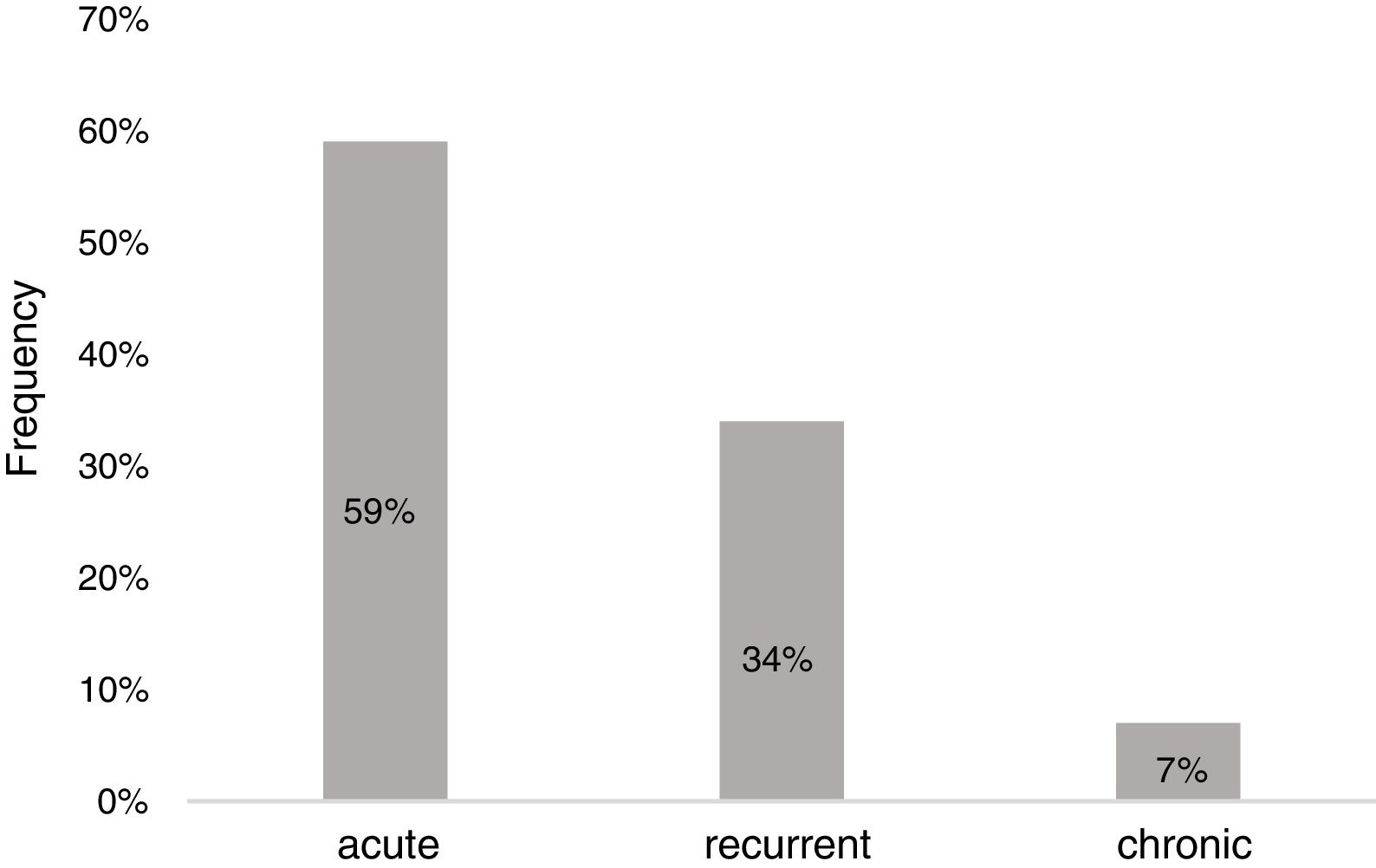
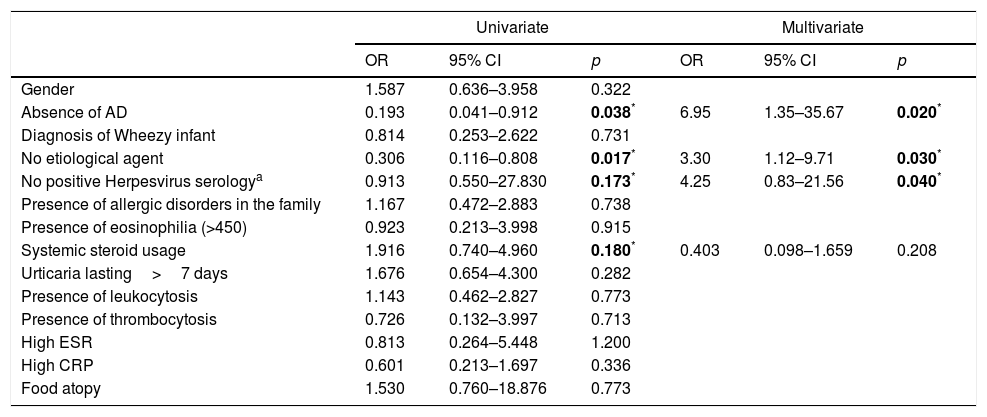
ResultsDemographicsEighty-three preschool children with AU [2.12 (1.27–3.39) years old, male 59%] were included in the study (Table 1). The median duration of wheals was five days (IQR: 3–10), whereas 28 children (34%) had urticaria lasting longer than seven days. Twenty-three patients (28%) had accompanying angioedema, and three patients had anaphylaxis. Forty-nine children (59%) had urticaria only once, 28 (34%) had recurrence, and six of the patients (7%) experienced chronicity (Fig. 1).
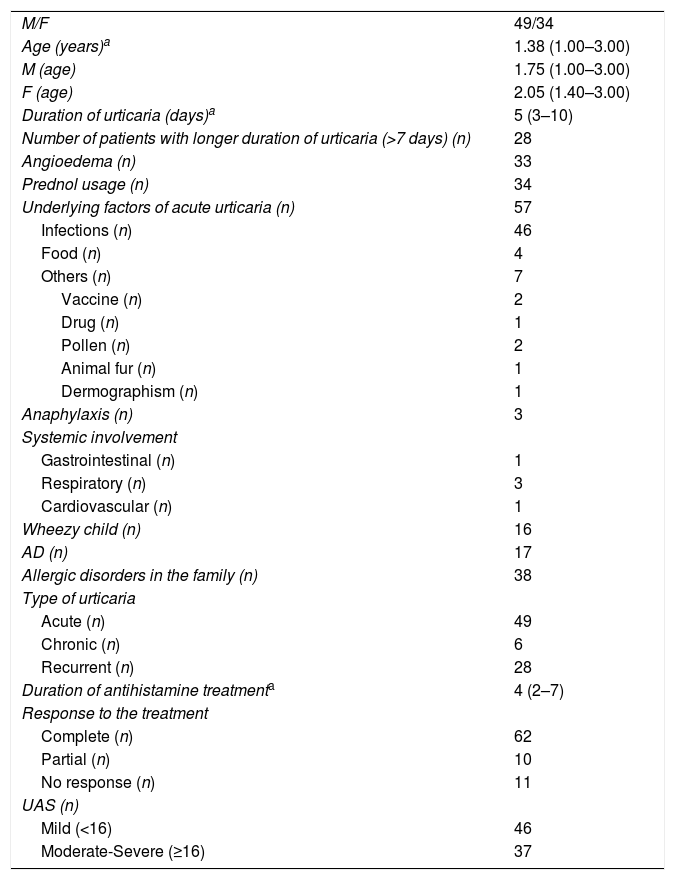
Demographic characteristics of the study population.
| M/F | 49/34 |
| Age (years)a | 1.38 (1.00–3.00) |
| M (age) | 1.75 (1.00–3.00) |
| F (age) | 2.05 (1.40–3.00) |
| Duration of urticaria (days)a | 5 (3–10) |
| Number of patients with longer duration of urticaria (>7 days) (n) | 28 |
| Angioedema (n) | 33 |
| Prednol usage (n) | 34 |
| Underlying factors of acute urticaria (n) | 57 |
| Infections (n) | 46 |
| Food (n) | 4 |
| Others (n) | 7 |
| Vaccine (n) | 2 |
| Drug (n) | 1 |
| Pollen (n) | 2 |
| Animal fur (n) | 1 |
| Dermographism (n) | 1 |
| Anaphylaxis (n) | 3 |
| Systemic involvement | |
| Gastrointestinal (n) | 1 |
| Respiratory (n) | 3 |
| Cardiovascular (n) | 1 |
| Wheezy child (n) | 16 |
| AD (n) | 17 |
| Allergic disorders in the family (n) | 38 |
| Type of urticaria | |
| Acute (n) | 49 |
| Chronic (n) | 6 |
| Recurrent (n) | 28 |
| Duration of antihistamine treatmenta | 4 (2–7) |
| Response to the treatment | |
| Complete (n) | 62 |
| Partial (n) | 10 |
| No response (n) | 11 |
| UAS (n) | |
| Mild (<16) | 46 |
| Moderate-Severe (≥16) | 37 |
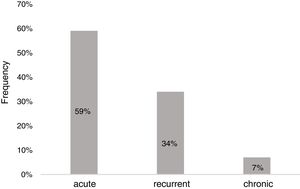
Of the 83 patients, 16 (19.3%) had a history of recurrent wheezing, and 17 (20.5%) had atopic dermatitis. The presumptive cause was determined in 68.7% (n=57) of the patients, and infections were the most frequent cause of AU (n=46, 80.7%) (Fig. 2). Other identified underlying factors are listed in Table 1.
Infectious etiologyForty-six children (55.4%) had various symptoms of infections, however microbiological causes remained unknown in half of the cases. Apparent upper respiratory tract infection (URTI) (n=44, 97.6%) was the most common type of infection. Infectious microorganism was shown in 23 out of 46 patients (50%). The remaining 23 patients without positive serology or culture had symptoms such as fever, cough, nasal discharge, sneezing, sore throat, and diagnosed as URTI or lower respiratory tract infection according to the symptoms and physical examination. HSV1 (n=13); EBV (n=4), Group A Beta-hemolytic Streptococcus (n=4), and Mycoplasma pneumonia (n=2) were identified in serum or throat swab samples (Table 2). HSV1 was significantly isolated in patients with acute single-episode urticaria (p=0.042). No vesicular lesions were observed on skin or mucosal surfaces of patients with positive HSV type 1 serology.
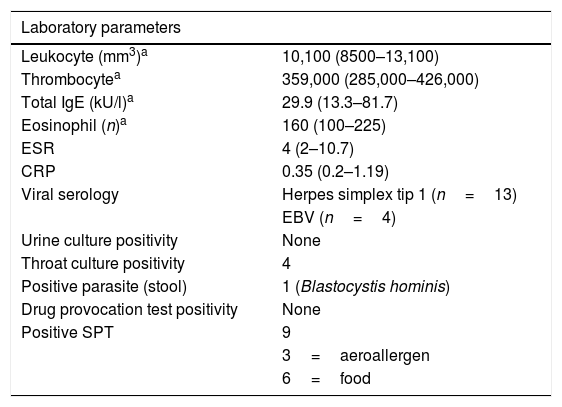
Laboratory parameters of the study population.
| Laboratory parameters | |
|---|---|
| Leukocyte (mm3)a | 10,100 (8500–13,100) |
| Thrombocytea | 359,000 (285,000–426,000) |
| Total IgE (kU/l)a | 29.9 (13.3–81.7) |
| Eosinophil (n)a | 160 (100–225) |
| ESR | 4 (2–10.7) |
| CRP | 0.35 (0.2–1.19) |
| Viral serology | Herpes simplex tip 1 (n=13) |
| EBV (n=4) | |
| Urine culture positivity | None |
| Throat culture positivity | 4 |
| Positive parasite (stool) | 1 (Blastocystis hominis) |
| Drug provocation test positivity | None |
| Positive SPT | 9 |
| 3=aeroallergen | |
| 6=food | |
CRP levels [median=0.20mg/L (IQR: 0.10–0.24), (p=0.021)] and platelet counts [median=243.000/mm3 (IQR: 202.500–340.250), (p=0.027)] were lower in patients who progressed to chronic urticaria than the acute/recurrent type. In addition, a positive relation between CRP level [median=0.59mg/L (IQR: 0.24–1.21)] and AU was determined (r=0.262, p=0.022).
Urticaria activity score (UAS)The median of UAS was 15 (8–21), and 55.4% of children (n=46) had mild urticaria (UAS<16). Higher UAS, indicative of an increased disease severity, was associated with longer duration of urticaria (r=0.578, p<0.001) but not with recurrent urticaria (r=0.005, p=0.966) or progression to chronic urticaria (r=0.044, p=0.69).
Age groupsWe divided the study population into two subgroups as below (n=38, 65.7% male) or above two years old (n=45, 53.3% male). The co-existence of angioedema with urticaria was observed in twenty-three children, and 87% of them (n=20) were under two years old (p=0.001). Angioedema involved lips in 47.8% of the cases (n=11), followed by extremities (n=7, 30.5%). All patients with food allergy were under two years old (p=0.006).
Progression to chronic/recurrent urticariaOverall, chronicity or recurrence was accounted for in 41% (n=34) of the study group. Recurrent urticaria (n=28) occurred more often in the patients without an identifiable etiology (n=26) (p=0.008), and in patients with negative Herpesvirus serology (p=0.032). Urticaria developed only once in children with positive Herpesvirus serology (p=0.027). We encountered that progression toward recurrence was observed in patients who experienced first urticaria onset in summer (p=0.008). In contrast, patients with first onset in winter had no recurrence (p=0.004). Furthermore, positive Herpesvirus serology was significantly documented in patients with urticaria onset in winter (p=0.036).
As was expected, urticaria lasted more than seven days in patients with chronicity (r=0.293, p=0.007). A positive relationship was determined between the duration of urticaria and chronicity (r=0.301, p=0.006). Intestinal parasite was identified in one patient, in whom Blastocystisi hominis was isolated in stool sample, and urticaria occurred only once in that case. Aeroallergen or food sensitizations were not found to be related to recurrence or chronicity.
Risk factorsIn univariate and multivariate analysis, the independent risk factors for the recurrence were the absence of AD (OR: 6.95, 95% CI: 1.35–35.67, p=0.020), negative Herpesvirus serology (OR: 4.25, 95% CI: 0.83–21.56, p=0.040), and unknown etiology (OR: 3.30, 95% CI: 1.12–9.71, p=0.030). However, we were not able to demonstrate any risk factors for chronicity (Table 3).
Predictive factors for recurrence following first onset of acute urticaria.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 1.587 | 0.636–3.958 | 0.322 | |||
| Absence of AD | 0.193 | 0.041–0.912 | 0.038* | 6.95 | 1.35–35.67 | 0.020* |
| Diagnosis of Wheezy infant | 0.814 | 0.253–2.622 | 0.731 | |||
| No etiological agent | 0.306 | 0.116–0.808 | 0.017* | 3.30 | 1.12–9.71 | 0.030* |
| No positive Herpesvirus serologya | 0.913 | 0.550–27.830 | 0.173* | 4.25 | 0.83–21.56 | 0.040* |
| Presence of allergic disorders in the family | 1.167 | 0.472–2.883 | 0.738 | |||
| Presence of eosinophilia (>450) | 0.923 | 0.213–3.998 | 0.915 | |||
| Systemic steroid usage | 1.916 | 0.740–4.960 | 0.180* | 0.403 | 0.098–1.659 | 0.208 |
| Urticaria lasting>7 days | 1.676 | 0.654–4.300 | 0.282 | |||
| Presence of leukocytosis | 1.143 | 0.462–2.827 | 0.773 | |||
| Presence of thrombocytosis | 0.726 | 0.132–3.997 | 0.713 | |||
| High ESR | 0.813 | 0.264–5.448 | 1.200 | |||
| High CRP | 0.601 | 0.213–1.697 | 0.336 | |||
| Food atopy | 1.530 | 0.760–18.876 | 0.773 | |||
The numbers in bold and with asterisk sign have been marked to indicate “statistically significant p values (p <0.05)”.
AU is frequently seen in pre-school children, but little is known about the underlying factors and prognosis relevant to this age group.4 In this study, we focused on the triggering factors and identified the independent predictors that can be used for the estimation of progression to chronic and recurrent urticaria in small children.
The underlying etiology was found in nearly 70% of the patients in contrast to the previous studies having higher ratios.5 Infection was the most frequently determined inciting reason that constituted half of the identifiable etiologies, a fact stated in several reports,4,9 which was also accompanied by the increased CRP levels and thrombocyte counts. The outcome of our study in regard to the higher CRP levels in AU was in agreement with a previous study, which reported that higher values of APRs were observed in acute urticaria rather than the chronic type.13 High CRP levels in single acute urticaria were possibly due to the significant correlation of infections detected at the time of the acute attacks. URTI was the leading infection that the physicians diagnosed.14 The study of Konstantinou et al. revealed that the seasonal pattern of AU attacks coincided with the respiratory tract infection outbreaks.3 According to our study, HSV1 was the mostly isolated infectious agent followed by EBV, group A beta hemolytic streptococcus, and Mycoplasma pneumonia. In recent publications, HSV has been incriminated as an important causative agent in urticaria.9 It has been demonstrated that HSV has the capability of driving the immune system toward the allergic pathway,15 and therefore determination of HSV as the most frequently isolated causative agent in the current study is in agreement with that outcome.
According to the current study, approximately 41% of the patients progressed to chronicity and recurrence. There were few reports regarding the prognosis of AU both in adults and children,5,16 and it was indicated that this ratio was 20–30% in children.5,17 Recurrent urticaria was observed mainly in children, who experienced the first onset in summer, and which was less associated with infections. Moreover, positive Herpesvirus serology as etiology was mostly identified in patients with single-episode AU in winter. As a result of the multivariate analysis, the absence of AD, negative Herpesvirus serology and unknown etiology were shown to be the independent risk factors for recurrence. Additionally, all of the six patients who progressed to chronicity had negative Herpesvirus serology in the current study. These outcomes contradict previous studies speculating that viral infections are the cause of the flare-ups in recurrent urticaria.9,17 On the other hand, the study by Legrain et al. reported a similar outcome that the underlying etiology remained unknown in recurrent or chronic urticaria.18 Possible reasons for chronic and recurrent urticaria, as we have seen in our study, are not only explained by the isolation of infectious agents, but are also believed to have a more complex pathogenesis.19 Future research will help to elucidate the conflicting results of the effects of the viral agents on the prognosis of AU in a small pediatric age group. According to the current study, the absence of AD was a risk factor for recurrence. The use of antihistamines and topical corticosteroids intermittently in patients with AD may be a reason for this outcome in recurrent urticaria. In previous studies, non-steroidal anti-inflammatory drug hypersensitivity, food allergy, the initiation of treatment after one week, and the requirement of medications other than antihistamines have been identified as poor prognostic factors for AU.16,20 However, here we did not observe find those parameters to be the risk factors for recurrence or chronicity. To the best of our knowledge, this is one of the very few studies regarding the prognosis of AU and the risk factors in progression toward recurrence and chronicity in small children. For this reason, we strongly believe that the current study may guide physicians for constituting a new perspective during the follow-up of children with AU.
UAS is a scoring system developed for chronic urticaria, which is consistent with disease activity, and correlates with response to therapy.21 Although designed for chronic urticaria, Liu et al. used this scoring system for AU.22 Based on the literature, we implemented UAS to our patients and demonstrated a positive correlation with a long-lasting duration of AU in concordance with previous reports.22 As the severity of AU increases, so the disease lasts longer, therefore the scoring of AU with UAS may influence the treatment and the follow-up preferences of physicians.
In childhood, virtually ten percent of urticaria coexists with angioedema.22 The most common reasons for angioedema in children were identified as food, insect bite, infections, and antibiotics.23 A strong association between angioedema, atopic dermatitis, and food allergy was revealed in small children.5 However, such a clinical relation was not demonstrated in our study. We observed that most of the patients with angioedema were under two years old. This finding was in total agreement with Mortureux et al., who reported that 60% of children aged one to 36 months with AU exhibited angioedema.5
Previously, IgE-mediated AU was reported in half of the patients,24 but in recent studies 3–10% of cases had IgE-mediated etiology.14,25 The cause of AU was shown to be hypersensitivity to either foods or aeroallergens in nearly 10% of our patients. AU induced by food is generally observed in children under two years old, due to the introduction of foods in the first years of life.23
There are some limitations to the present study. The infectious agents could not be determined in some patients due to the lack of the use of the respiratory viral panel, which might add additional information. The sample of the study population might be higher, however, we included all children younger than five years of age admitted within a one-year period. The major strengths of our study were the prospective design, the unbiased enrollment of patients and the evaluation of risk factors in proceeding to chronicity or recurrence.
The establishment of the precipitating factor in conjunction with the laboratory and clinical parameters may not be possible in some children with AU. Herpesvirus infections, especially HSV type 1, were identified as the most frequently encountered reason for AU in small children. However, atopy constituted only a small part of the identifiable reasons for AU. Pediatricians should take into account the recurrent nature of the disease in children with AU who had independent predictors for recurrence, including the unknown etiology, negative Herpesvirus serology, and the absence of AD, and should inform parents to prevent anxiety.
Author contributionPinar Gur Cetinkaya: Collected the data and wrote the manuscript.
Ozge Soyer: Collected the data and revised the manuscript.
Saliha Esenboga and Ozlem Teksam: Collected the data.
Umit Murat Sahiner: Completed the statistical analysis of the study.
Bulent Enis Sekerel: Revised the manuscript.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Right to privacy and informed consentAll parents provided informed consent.
Protection of human subjects and animals in researchThis study was completed in accordance with the ethical standards specified in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Hacettepe University.




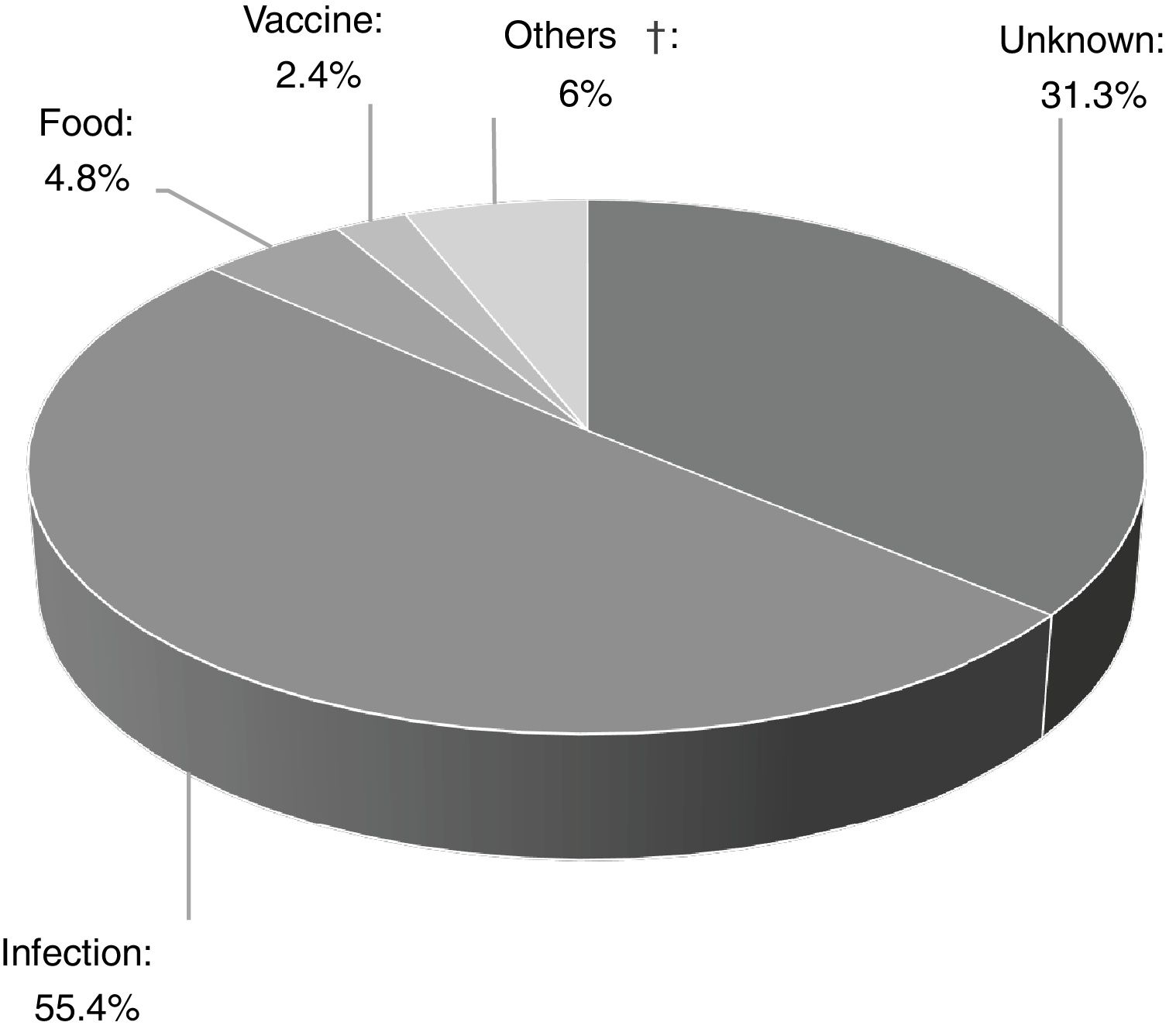
![Triggering factors of acute urticaria in the study group [Others†; animal fur (n=1), drugs. (n=1; mydriatic and cycloplegic eye drops), grass pollen (n=2), physical dermographism (n=1)]. Triggering factors of acute urticaria in the study group [Others†; animal fur (n=1), drugs. (n=1; mydriatic and cycloplegic eye drops), grass pollen (n=2), physical dermographism (n=1)].](https://static.elsevier.es/multimedia/03010546/0000004700000005/v1_201908090713/S0301054619300254/v1_201908090713/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
