INTRODUCTION
Cow's milk proteins are among the most common causes of food allergy in infants, and caseins are probably the main allergens1 The existence of high degree of cross-reactivity between milk caseins from different animals have been reported2-5.
CASE REPORT
A 2-year-old boy developed urticaria and angioedema 15 minutes after eating sheep cheese. Afterwards, experienced contact urticaria touching sheep cheese. He tolerated cow's milk and cow's milk dairy products. He had never ingested milk or milk derivatives from sheep or goat.
MATERIALS AND METHODS
Skin tests
Skin prick test (SPT) were carried out using whey fractions of cow's milk, whole milk and caseins (Sigma Chemical Co.,USA) from goat, sheep and cow. We also performed SPT with Lactococcus alactis and Streptococcus termophilus (used in cheese manufacture). Prick-by-prick test with cheese made from cow, sheep and goat and their corresponding whole milk were also performed.
Total and specific IgE determination
Total serum IgE and specific IgE (CAP Pharmacia) to cow's milk proteins, whole cow's milk and sheep's milk were determinated. Specific IgE against caseins and whole milk from the three different species were determined by ELISA technique using a concentration of 10 μg/ml and 1:10 serum dilution as previously described6.
ELISA inhibition
Inhibition of the IgE binding to bovine casein was tested for caseins and whole milk from all three species. The concentration which produced 50 % inhibition was calculated.
SDS-PAGE and Immunoblotting
The proteins of three types of commercial purified caseins and whole milk from three species were separated by SDS-PAGE (sodium dodecylsulphate-poyacrylamide gel electrophoresis), following the Laemmli method7.
After SDS-PAGE, transfer of proteins from the gel onto the nitrocellulose membrane was carried out according to Towbin et al8. The membrane was then incubated with the patient serum. After washing, the membrane was incubated with antihuman IgE conjugated with peroxidase.
RESULTS
Skin tests
SPT were positive to sheep's milk and goat and sheep caseins and negative to all cow's milk proteins and whole cow's and goat's milk.
Prick-by-prick tests were positive to goat's and sheep's cheese and negative to cow's cheese.
Total and specific IgE determination
Total serum IgE was 40 KU/l. Specific IgE antibodies by CAP were negative (< 0.35 KU/l) to all cow's milk proteins and positive to sheep's milk (2.85 KU/l). IgE ELISA results were positive (> 0.250 expressed in optical densities) to sheep casein (0.548 OD), goat's milk (0.262 OD) and goat casein (0.317 OD).
ELISA inhibition
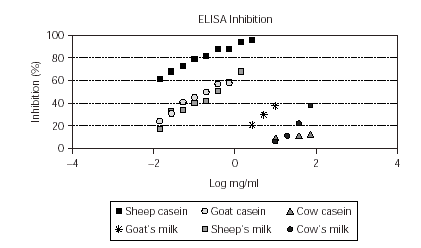
When sheep casein was used in the solid phase, sheep's milk and goat and sheep casein were able to inhibit > 50 % the specific IgE binding to sheep casein. The lowest inhibition was observed with cow casein (12.4 %) (fig. 1).
Figure 1.--ELISA Inhibition of sheep casein (solid phase) using caseins and whole milks from sheep, goat and cow as inhibitor allergens.
SDS-PAGE and Immunoblotting
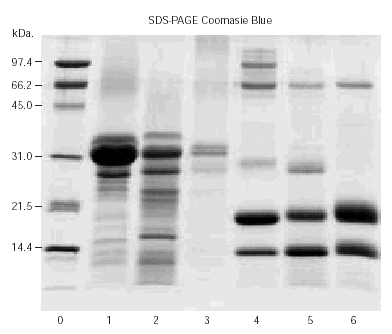
The results of SDS-PAGE are shown in figure 2. Bands corresponding with 34 and 24 kDa were observed in casein lanes. Three bands that may correspond to α-lactalbumin, β-lactoglobulin and serum albumin were found in lanes corresponding to whole milks.
Figure 2.--SDS-PAGE of sheep casein (lane 1), goat casein (lane 2), cow casein (lane 3), sheep's milk (lane 4), goat's milk (lane 5) and cow's milk (lane 6). Lane 0: molecular weight markers.
Figure 3 shows the results of Immunoblotting analysis. Circulating IgEs of patient recognize only one band in caseins of sheep and goat lanes with molecular weight about 33-34 kDa.
Figure 3.--IgE immunoblots of sheep casein (lane 1), goat casein (lane 2), cow casein (lane 3), sheep's milk (lane 4), goat's milk (lane 5) and cow's milk (lane 6).
FOLLOW UP
The patient was able to eat cow milk dairy products and after one year of following up the tolerance persists.
DISCUSSION
Selective allergy to sheep's milk products in the absence of cow's milk allergy is quite rare3-5. Reports published to date are few. The first article published in 19953 showed the existence of cross-reactivity between sheep's and goat's casein, but no with cow casein demonstrated by RAST inhibition. The last one appeared in 19995 included in its study SDS-PAGE of milk caseins from cow, sheep and goat and immunoblotting which identifies a number of IgE-binding bands in goat and sheep casein but not in cow casein.
We report on a patient with allergy selective caused by sheep's and goat's milk proteins, but not to cow's milk proteins. We demonstrated by in vivo and in vitro tests that sheep casein is probably the main allergen causing sensitization in this patient.
The mayor cow's milk antigens are the whey proteins α -lactalbumin, β -lactoglobulin, bovine serum albumin and the caseins9. Recent studies suggest that casein may be the main allergen implicated in the IgE- mediated milk allergy, both in children1 and adults10. Casein is composed of different proteins with several sequences: α-(subdivided into two fractions: as1- and as2-), β-, κ- and γ-caseins. From these, α-casein is quantitatively more important and it is one of the major allergens of cow's milk11. The analysis of the amino acid sequences of the four fractions of caseins from ruminant species evidence high sequence homologies varying from 80 % to more than 90 %; this large identity facilitates immunological cross- reactivity2.
However, the results of the ELISA inhibition suggest that sheep casein show a high degree of cross-reactivity with goat casein but not with cow casein in this case. Nevertheless, this child never showed any clinical reaction to cow's milk, and skin prick-by-prick test with whole cow's milk and its casein elicited no reaction. Immunoblotting showed IgE-binding bands of similar molecular weight in lanes corresponding with sheep and goat caseins, whereas any band was observed in cow casein. All of our results point out a selective immunological recognition to specific epitopes in caseins from sheep and goat but non to casein from cow explaining the clinical tolerance to cow milk products in this patient. Few structural variants in the amino acids sequence may affect the allergenicity of caseins.
None of the studies published before include a follow of patients. However, it would be necessary a continuous evaluation of the patient because there is a high degree of cross-reactivity between milk proteins from different mammals (not only caseins but also other milk proteins) and the possibility of new sensitisations. We recommend periodic allergological controls with the purpose of detecting new sensitizations to cow's and other mammal's milk and dairy products as soon as possible. We allowed the ingestion of milk and derivatives from cow but not from another mammals. It is very important make sure that the tolerance persists.
In conclusion, our patient was diagnosed from selective allergy caused by casein from sheep's and goat's milk.