Background. Portal hypertension is a clinical syndrome associated with the development of a hyperdynamic circulation and gastroesophageal varices.
Aim. To evaluate the antioxidant effect of N-acetylcysteine on portal hypertensive rats.
Material and methods. Portal hypertension was induced by partial portal vein ligation (PPVL). Oxidative damage in the stomach was measured by lipoperoxidation trough thiobarbituric acid reactive substances (TBARS) and antioxidant enzyme activity; we also evaluated nitrates and nitrites level and histology stained by hematoxylin-eosin. We performed evaluation of portal pressure and measurement of vessels diameter. Liver damage was evaluated by measuring hepatic enzymes. The animals were divided in four experimental groups (n = 6): Sham-operated (SO), SO + NAC, Partial portal vein ligation (PPVL) and PPVL + NAC. N-acetylcysteine (10 mg/kg ip) was administered daily for 7 days and started 8 days after surgery.
Results. The portal hypertensive group showed an increase in portal pressure, vessels diameter, levels of TBARS and nitrates and nitrites when compared to SO group. These values were accompanied by a decrease in superoxide dismutase (SOD) and glutathione peroxidase (GPx) antioxidant enzyme activity. Histology showed dilated vessels in the gastric mucosa in the PPVL group. NAC was able to decrease portal pressure values, vessels diameter, TBARS and also nitrates and nitrites levels when compared to PPVL group. Furthermore, PPVL+NAC group presented an increase in SOD and GPx activity. N-acetylcysteine attenuated damage in gastric mucosa.
Conclusion. Oxidative stress is associated with portal hypertension and that antioxidant NAC is able to minimize damages of PPVL in rats.
Portal hypertension (PH) is a clinical syndrome which is hemodynamically defined as a pathological increase in portal pressure, with consequent formation of porto-systemic collaterals that deviate blood flow from the portal system directly to systemic circulation.1
In clinical PH, the emergence of this collateral circulation leads to dilatation of vessels especially in the stomach and esophagus, leading to portal hypertensive gastropathy (PHG), which is characterized by vasodilation in the gastric submucosa. These hemodynamic disturbances lead to bleeding due to the progressive dilatation of vessels that break and lead to hemorrhage.1
Partial portal vein ligation (PPVL) is the most used experimental model to study the pathophysiology of prehepatic portal-hypertension gastropathy. It has been developed by Sikuller, et al. and several experimental studies demonstrated that PPVL manifests gastric abnormalities equivalent to PHG in humans.2 One week after PPVL the operated animals already develop PHG and approximately 100% of these animals present portosystemic shunting and hyperdynamic circulation, in other words, collateral vessels.3
Oxidative stress has been appointed as trigger factor to the progression of several diseases. In PH, it is related to the development of the hyperdinamic circulation and to the overproduction of nitric oxide (NO).4 Recently, increased NO serum levels were found in patients with portal hypertensive gastropathy, being implicated in the pathogenesis of this disease.5 The enhanced synthesis of NO induces peroxynitrite formation by reaction to other reactive species of oxygen, therefore increasing oxidative damage to the gastric mucosa.6 N-Acetylcysteine (NAC) is a reliable, inexpensive and well-tolerated antioxidant, with a well-known mechanism. Treatment with NAC was found to improve the levels of glutathione, the major endogen antioxidant of humans. NAC can also decrease the bioavailability of nitric oxide by binding its thiol component with it and forming nitrotiol.7 The primary aim of this study was to evaluate the antioxidant action of NAC by assessing liver integrity, stomach lipoperoxidation through thiobarbituric acid reactive substances (TBARS), the activity of antioxidant enzymes superoxide dismutase and glutathione peroxidase, as well as perform the measurement of nitrites and nitrates and histological analysis in stomach of rats submitted to the experimental model of PPVL.
Material and MethodsEthicsThe experimental procedures complied with the rules established by the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
AnimalsTwenty-four male Wistar rats weighing 250 g were used. They were obtained from State Foundation of Production and Research in Health (FEPPS), Porto Alegre, RS. They were kept at the vivarium of the Lutheran University of Brazil in plastic boxes measuring 47 x 34 x 18 cm lined with wood chips, under a 12-h dark/light cycle (light from 7 a.m. to 7 p.m.) at a temperature of 22 ± 4 oC. The rats were fed 16 g per animal/day on rat chow (Purina-Nutripal, Porto Alegre, RS, Brazil) and had water ad libitum.
Groups and treatment protocolsThe animals were divided in four experimental groups (n = 6): Sham-operated (SO), SO + NAC, partial portal vein ligation (PPVL), and PPVL + NAC. N-Acetylcysteine (Sigma Chemical Co., St. Louis, MO, USA; CAS registry number 616-91-1) was dissolved in 0.6 mL of 0.9% NaCl and injected in a dose of 10 mg/kg intraperitonially of animal weight. The animals of the PPVL and SO groups received the same volume of vehicle, without NAC, for the same period as the ones in PPVL+NAC and SO+NAC groups. It was administered daily starting on day 8 after surgery and extended for 7 days.
Portal hypertension inductionThe animals were anesthetized with ketamine hydrochloride (100 mg/kg ip) and xylazine hydrochloride (50 mg/kg ip). After a medium incision in the abdomen, bowels were gently withdrawn on a humidified gauze with saline and the portal vein was isolated. A 20 g needle was placed on the portal vein and both were tied up using a 3.0 silk yarn, the needle being gently withdrawn after ligation. Afterwards, we tested for the absence of portal vein thrombosis by manipulating the spot.2 The sham-operated group was submitted to the same procedure, although their portal veins did not undergo partial portal vein ligation.
EuthanasiaOn day 15 after the surgery the animals were anaesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (50 mg/kg ip). Through a heparinized capillary, a blood sample was taken from the retro-orbital plexus in order to assess liver integrity by aspartate aminotransferase (AST), alanine aminotranferase (ALT), and alkaline phosphatase (FA).8
For the determination of AST and ALT in the plasma we used a commercial enzymatic method (Boehringer Mannheim, Germany). Thus, the enzymatic activity of AST and ALT was obtained by measuring kinetics at 567 nm. For determination of FA activity in plasma we employed an automated enzymatic method. For this purpose, we used as a substrate para-nitrophenyl and water, that forms para-nitrophenol, a yellow compound with a maximum absorbance of 400 nm
The abdomen was shaved, followed by laparotomy, and the stomach was removed for histological analysis to evaluate the tested segment; the rest was frozen −80 oC for later biochemical analysis. The animals were killed by exsanguination under deep anaesthesia.9
Measurement of portal pressurePortal pressure was measured in mmHg on a Poligraph 2006 (Lettica Scientific Instruments, Barcelona, Spain) by cannulation of the mesenteric vein with a catheter.2
Stomach homogenatesThe stomachs were cut with scissors and weighed. Five millilitres of phosphate buffer (140 mM KCL, 20 mM phosphate, pH 7.4) per tissue gram was added, and the tissue was homogenized in an Ultra Turrax (IKA-WERK) for 40 seconds at 4 oC. Next, it was centrifuged for 10 minutes at 4.000 rpm (2150.4 x g) (SORVALL RC-5B Refrigerated Superspeed Centrifuge). The supernatant was pipetted into Eppendorf flasks, and the precipitate was discarded. The samples were stored again at −80 °C for posterior analyses.
ProteinWe used the Bradford method to quantify protein, with bovine albumin as the standard (SIGMA®). The samples were measured spectrophotometrically at 595 nm, and values expressed in mg/mL were used to calculate values of TBARS (thiobarbituric acid-reactive substances) and antioxidant enzymes.10
Stomach liperoxidationThe amount of aldehydes generated by lipid peroxidation is measured by the TBARS method, which measures the amount of substances reacting with thiobarbituric acid. The samples were incubated at 100 oC for 30 min after addition of 500 µL of 0.37% thiobarbituric acid in 15% trichloroacetic acid and centrifuged at 3,000 rpm (1612.8 x g) for 10 min at 4 oC. Absorbance was determined spectrophotometrically at 535 nm and values were expressed in nmol/mgprot.11
Nitrates and nitrites levelsThe levels of nitrates and nitrites were measured by the reaction of the samples with Griess reagent. Aliquots of 50 µL were incubated with enzyme cofactors and nitrate reductase for 30 min at room temperature for the conversion of nitrate to nitrite. The nitrite formed was then analysed by reaction with the Griess reagent, forming a coloured compound that was measured by spectrophotometer at a wavelength of 540 nm.12
Antioxidants enzyme analysesThe analysis of superoxide dismutase (SOD) in stomach is based on the inhibition of the reaction of the superoxide radical with adrenaline, values expressed in U/mgprot.13 Glutathione peroxidase (GPx) activity is based on the consumption of NADPH in the reduction of oxidized glutathione and values were expressed in nmol/mgprot.14
Histologycal analysesFor histological evaluation, stomach fragments slides were stained with hematoxylin and eosin and subsequently assessed by a single pathologist in blind fashion. The vessels caliber was measured in pixels.
Statistical analysisAll data are presented as means ± SE. Statistical significance was calculated using Graphpad Instat, version 3.0 for Windows. Variance analysis (ANOVA) and Student-Newman-Keuls were used for multiple analysis, and the level of significance was 5% (P < 0.05).
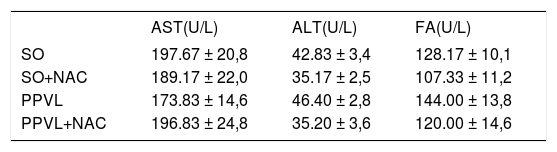
ResultsTransaminase activitiesNo significant differences were found for any of the tested parameters across the four groups sampled (Table 1).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on serum AST, ALT and FA activities.
| AST(U/L) | ALT(U/L) | FA(U/L) | |
|---|---|---|---|
| SO | 197.67 ± 20,8 | 42.83 ± 3,4 | 128.17 ± 10,1 |
| SO+NAC | 189.17 ± 22,0 | 35.17 ± 2,5 | 107.33 ± 11,2 |
| PPVL | 173.83 ± 14,6 | 46.40 ± 2,8 | 144.00 ± 13,8 |
| PPVL+NAC | 196.83 ± 24,8 | 35.20 ± 3,6 | 120.00 ± 14,6 |
AST: aspartate aminotransferase. ALT: alanine aminotransferase. FA: alkaline phosphatase. SO: Sham-operated group. SO+NAC: Sham-operated treated with NAC group. PPVL: partial portal vein ligation group. PPVL + NAC: partial portal vein ligation group treated with NAC. There was no statistic difference between the sampled groups.
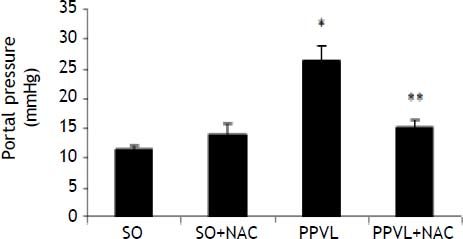
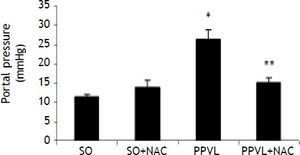
There was a statistically significant increase in portal pressure values in the PPVL group as compared to SO (P < 0.001), and in the NAC-treated group these values were significantly decreased (P < 0.001) (Figure 1).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on portal pressure. SO: Sham-operated group. SO + NAC: Sham-operated treated with NAC group. PPVL: partial portal vein ligation group. PPVL + NAC: Partial portal vein ligation group treated with NAC.* P < 0.001 against sham-operated. ** P < 0.001 against PPVL (n = 6).
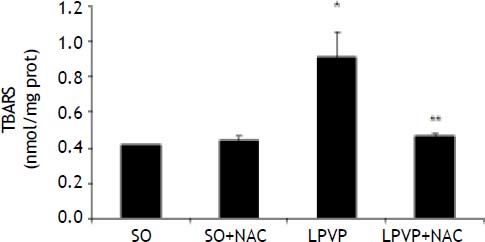
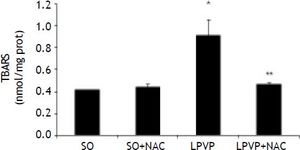
TBARS values in the stomach were found to be significantly increased in the PPVL as compared to the SO group (P < 0.001). In the treated PPVL group those values were significantly decreased, getting close to those of the sham-group (P < 0.001) (Figure 2).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on gastric mucosa TBARS. SO: Sham-operated group. SO + NAC: Sham-operated treated with NAC group. PPVL: partial portal vein ligation group. PPVL + NAC: partial portal vein ligation group treated with NAC. * P < 0.001 against sham-operated. ** P < 0.001 against PPVL (n = 6).
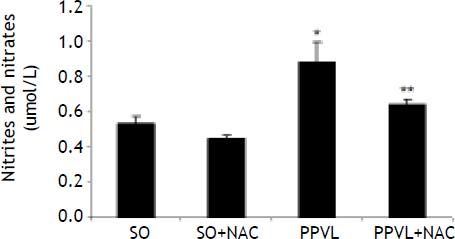
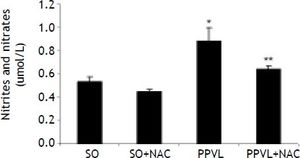
NO values in the stomach were found to be significantly increased in the PPVL as compared to the SO group (P < 0.01). In the NAC-treated PPVL group those values were significantly decreased (P < 0.05) (Figure 3).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on nitrates and nitrites level. SO: Sham-operated group. SO + NAC: Sham-operated treated with NAC group. PPVL: partial portal vein ligation group. PPVL + NAC: partial portal vein ligation group treated with NAC.* P < 0.01 against sham-operated. ** P < 0.05 against PPVL (n = 6).
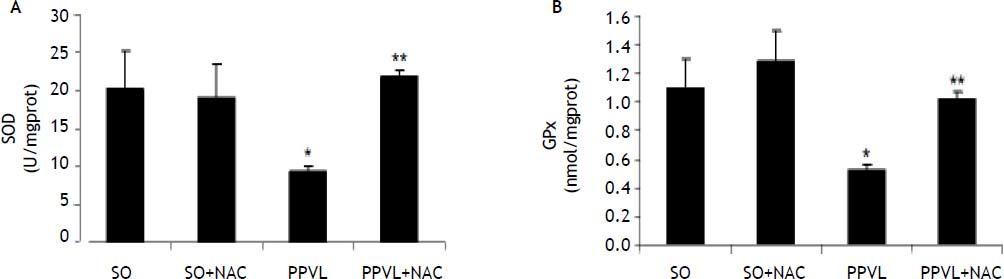
SOD activity was significantly decreased in PPVL animals as compared to SO (P < 0.05), and NAC treatment caused a significant increase in SOD values as compared to the PPVL group (P < 0.05) (Figure 4A).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on gastric mucosa SOD (A) and GPx (B) enzyme. SO: Sham-operated group. SO + NAC: Sham-operated treated with NAC group. PPVL: partial portal vein ligation group. PPVL + NAC: Partial portal vein ligation group treated with NAC. A. * P < 0.05 against sham-operated. ** P < 0.05 against PPVL (n = 6). B. * P < 0.05 against sham-operated. ** P < 0.05 against PPVL (n = 6).
GPx activity showed the same behavior as SOD, with a significant decrease in the PPVL group as compared to SO (P < 0.05). NAC was able to reverse this effect, as there was a significantly increase in GPx values in NAC-treated animals as compared to the values obtained for the PPVL group (P < 0.05) (Figure 4B).
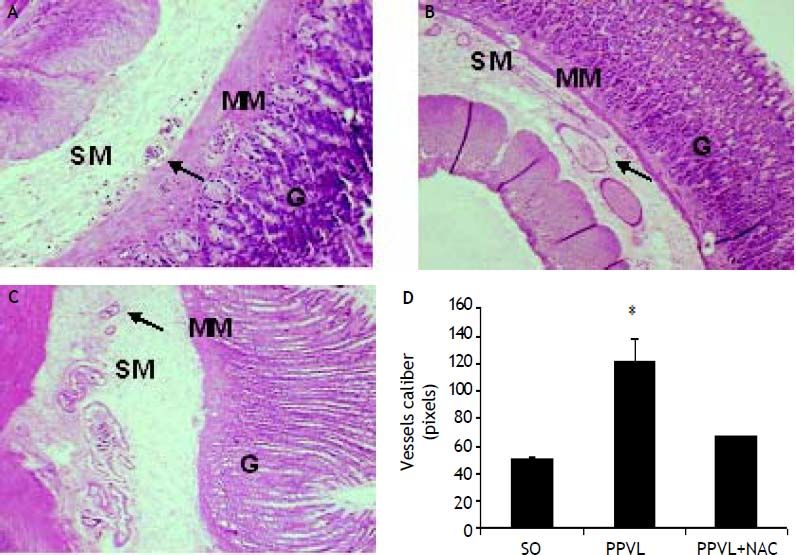
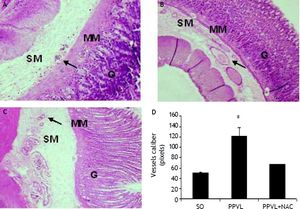
Histological analysisHematoxylin-eosin staining evidenced modifications in the normal architecture of the stomach, with edema and vasodilation in the PPVL group, and an attenuation of most changes in NAC-treated animals (Figures 5A-5C). PPVL animals presented an increase of vessels diameter (P < 0.001), and NAC-treated animals demonstrated a decrease of those values (P < 0.001), minimizing the damage and vasodilation in this experimental model (Figure 5D).
Effects of partial portal vein ligation (PPVL) and N-Acetylcysteine (NAC) administration on gastric mucosa histology (A-C) and vessels caliber (D). A. Sham-operated group (SO). B. Partial portal vein ligation group (PPVL). C. Partial portal vein ligation group treated with NAC (PPVL + NAC). Magnification of 40X. SM: submucosae. MM: muscular mucosa. G: glandular tissue. Hematoxylin-eosin histology evidenced modifications of the normal architecture, with vasodilation in the gastric PPVL group, pointed by the arrows. NAC was able to ameliorate tissue integrity by minimizing vasodilation. D. Sham-operated group (SO). Partial portal vein ligation group (PPVL). Partial portal vein ligation group treated with NAC (PPVL + NAC). * P < 0.001 against sham-operated. ** P < 0.001 against PPVL.
Portal hypertension (PH) is associated with the development of a hyperdynamic circulation, which is characterized by vasodilation and the increase of cardiac output and/or blood flow. The development of portal-systemic collaterals is a vascular response to the increased portal pressure.15
Gastroesophageal varices are the most prominent collaterals, which develop in order to diverse blood flow from the portal system and direct it to the systemic circulation. The vasodilation occurring in the stomach leads to portal hypertensive gastropathy.16
Oxidative stress leads to an imbalance in the redox status of the cell, either by excessive generation of reactive oxygen species (ROS) or a decrease in antioxidant enzymes.17 Nitric oxide is a central mediator of the vasoreactive and angiogenic abnormalities observed in portal hypertension.18 Investigators have concluded that enhanced NO synthesis contributes to the hyperdinamic circulation of PH, and therefore, oxidative stress.19
Many are the experimental models used to study the complications of intra, pre- and post-hepatic PH, and PPVL is among the pre-hepatic ones.3 In a pre-hepatic model, like the one used in this work, there is splenomegaly, splenic vessels dilation, and development of expressive collateral circulation that determines the emergence of gastro-esophageal varices, characterizing the portal hypertensive syndrome.20 In this experimental model, all the effects are related to pre-hepatic portal hypertension, since there is no damage to the liver of these animals.21
The fact that PPVL leads to oxidative damage was previously described by Fernando, et al., who stated that ROS generation may be intimately related to the hemodynamic alterations seen in portal hypertension.22
N-Acetylcysteine (NAC) has ability to support endogen antioxidants and modulate nitric oxide (NO) production during stressing situations, infections, toxic aggressions, and inflammatory conditions.23 In disorders where NO plays an important role, those properties are extremely important. The decrease of NO by NAC could minimize the oxidative stress in these disorders.
Recently, NAC was used in an experimental model of hepatic encephalopathy, a consequence of cirrhosis, with very promising results.24 However, studies evaluating the effect of NAC on antioxidant enzymes and histological parameters using the experimental model of PPVL are not found.
In the evaluation of liver enzymes, we confirmed previous studies which showed that PPVL does not effect any hepatocellular damage.18,21 This experimental model does not inflict liver damage.25
We observed a significant increase in portal pressure in the PPVL group. This demonstrates the effectiveness of this experimental model and confirms previous studies performed in our laboratory.18,21 NAC was able to reduce portal pressure, since it is able to modulate the production of nitric oxide.23
The overproduction of NO has a cytotoxic potential, enhancing mucosal injury (26). Previous studies performed by our group reported that the antioxidant treatment with glutamine and quercetin were able to reduce NO production, ameliorating the gastric mucosa integrity18,21 such as in this study.
We observed increased lipoperoxidation in PPVL animals, and NAC administration was associated with significant decreases in those values. Lipoperoxidation was evaluated previously in this model through F2–Isoprostanes measurement, and NAC treatment was able to improve this condition, just as it did in the present study.22
The increased lipoperoxidation was accompanied by a significant decrease in SOD and GPx activities, and NAC reverted this, increasing them. These enzymes are known to be inhibited by radical reaction products, which are overproduced on PH. SOD is inhibited by hydrogen peroxide,27 and GPx is inhibited by superoxide.28 NAC was able to restore both enzymatic activities, enhancing antioxidant activity. Such result corroborates to a previous study in which NAC treatment resulted in similar results.29
In the histological analysis, we observed a modification in the architecture of the gastric mucosa of PPVL rats, which showed vasodilation. We also measured the vessels caliber, and observed the presence of this abnormality. NAC was able to attenuate this histological change inflicted by PPVL, by decreasing this parameter in this experimental model.
Different authors have demonstrated that PPVL rats show abnormalities of the gastric microvasculature similar to those observed in humans with PH.3 Our group described previously that antioxidant treatment in experimental model of PPVL is able to ameliorate the gastric mucosa.18,21
The decrease in GPx and SOD activities in the gastric mucosa, accompanied by increased lipoperoxidation demonstrates the presence of oxidative damage in animals submitted to this experimental model of PPVL, with hemodynamic alterations such as increased portal pressure and vasodilation/edema in the gastric mucosa. The decrease in lipoperoxidation values associated with the increase in SOD and GPx activities evidence the effectiveness of N-Acetylcisteine in portal hypertension, possibly because of its antioxidant capacity.
In portal hypertensive gastropathy, it is important to define a suitable medication, able to minimize the oxidative damage and findings in the gastric mucosa. These are the main triggering events that lead to bleeding and anemia in clinical PHG, being them the major complications occurring in these patients. The use of an antioxidant therapy, capable of decreasing these complications is much valuable, in this study we observed N-Acetylcysteine acting in favor of minimizing all damage induced by PPVL in rats. Therefore, NAC can be beneficial in the treatment of portal hypertensive gastropathy.
ConclusionIn sum, NAC was able to minimize the damage to the gastric mucosa of animals submitted to the experimental model of PPVL, an animal model of pre-hepatic portal hypertension. This antioxidant was able to ameliorate rats with portal hypertension, by decreasing portal pressure and minimizing oxidative damage evaluated through TBARS, antioxidant enzymes SOD and GPx. N-Acetylcysteine also decreased the overproduction of nitric oxide in PPVL rats and also the vessels caliber, therefore minimizing the vascular dilation in this experimental model.
Abbreviations- •
ALT: alanine aminotransferase.
- •
ANOVA: one-way analysis of variance.
- •
AST: aspartate aminotransferase.
- •
FA: alkaline phosphatase.
- •
GPx: Glutathione peroxidase.
- •
NAC: N-Acetylcysteine.
- •
NO: nitric oxide.
- •
PH: portal hypertension.
- •
PHG: portal hypertensive gastropathy.
- •
PPVL: partial portal vein ligation.
- •
ROS: reactive oxygen species.
- •
SO: Sham-operated.
- •
SOD: superoxide dismutase.
- •
TBARS: thiobarbituric acid reactive substances.
We thank the staff at Laboratory of Experimental Hepatology and Physiology and Laboratory of Oxidative Stress and Antioxidants for their excellent scientific support.
Financial SupportSupported by grants from the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Foundation for research in the state of Rio Grande do Sul (FAPERGS), Fundo de Incentivo à Pesquisa e Eventos (FIPE) of the Hospital de Clínicas of Porto Alegre (HCPA-11-0293), and Laboratory of Experimental Hepatology and Gastroenterology (HCPA/UFRGS) of the Federal University do Rio Grande do Sul (UFRGS)/and Laboratory of Oxidative Stress and Antioxidants.