Introduction. Hydatid disease is a major health problem in some parts of the world. There are several non-operative and operative ways to treat hydatic disease. The aim of this retrospective study is to assess the rate of postoperative complications, mortality rate, hospitalization period, and recurrence for capsulorrhaphy method, and to compare it with other hydatic cyst management techniques.
Material and methods. An open surgical procedure using capsulorrhaphy technique was performed on 250 patients (130 men and 120 women) with uncomplicated hydatic cysts in the Northwest of Iran, between 1989 and 2011. Results. The patients stayed in the hospital between 4 and 10 days, with an average of 5 days. Of the patients, 233 (93.2%) were discharged without any complications, 9 (3.6%) developed a wound infection in the abdominal wall, and 7 (2.8%) developed pulmonary atelectasis. Those who developed an infection or an atelectasis stayed in the hospital for few more days for conservative treatments. One of the patients (0.4%) had external biliary fistula and bile leak, which was treated with surgery and Roux-en-Y cystojejunostomy. During follow-ups (13.2 ± 8.5 months), incisional hernias occurred in 5 (2%) patients and hydatid cyst recurrence affected 7 (2.8) patients. The mortality rate was zero amongst the studied patients.
Conclusion. Compared to other techniques in the literature, the results presented in the current work indicate that capsulor-rhaphy is an efficient method in terms of decreased postoperative complications, recurrence, and hospitalization period, and is a safe method with low morbidity and zero mortality rates.
There are six types of hydatid disease, of which four are considered the most hazardous to public health:1
- •
Echinococcus granulosus is the most common type, and causes cystic hydatid disease.
- •
Echinoccocus multilocularis is an uncommon and more aggressive type, giving rise to alveolar hydatid disease, and often mimics malignancy.2
- •
Echinococcus vogeli, or polycystic hydatid disease, is the most infrequent form with characteristics similar to cases 1 and 2.3
- •
Echinococcus oligarthrus, which causes polycystic echinococcosis.1
The Echinococcus granulosus is a cestode that grows and lays eggs in the small intestine of its host, usually a dog, and then transfers to an intermediate host, usually a sheep or human, through ingestion of vegetables contaminated with the primary host’s feces. Larvae of the parasite exit the egg in the duodenum and pass through the intestine to reach other organs.4 This disease is a major health problem in some parts of the world such as Eastern Europe, Mediterranean countries, South America and the Far East, including India.5 Hydatid disease is also endemic in East Azerbaijan, a province located in the Northwest of Iran, where this study was conducted. Nearly 50 to 70 percent of the cysts in the human body are found in the liver,6 mainly localized in the right lobe. A further 20 to 30 percent of cysts are found in the lungs. Cysts are not frequently seen in other organs such as the spleen, kidneys, heart, bones, and central nervous system.7
A liver cyst found in a patient from an endemic area is a symptom for a diagnosis of liver hydatid disease. Plain X-ray films showing calcification on the cyst wall are often used for cyst identification.8 Abdominal ultrasonography is commonly used for defining the number, site, dimensions and vitality of cysts.9,10 Gharbi, et al. used the morphologic appearance and structure of cysts in sonographic images to classify liver hydatid disease into five types,11 which is useful when evaluating treatment options.12 In Gharbi’s classification, type II and III indicate hydatid cysts, type I and V suggest hydatid cysts in endemic area, and type IV simulates a pseudotumor.13 Computed tomography (CT) scanning provides further details of a cyst, such as daughter cysts.8 Furthermore, in the cases where ultrasound examinations show a type IV sonographic pattern, CT can be used for a more accurate diagnosis.13 Serological studies, such as Casoni’s skin test and an indirect haemagglutination test, may also be used to verify the diagnosis for hydatid cysts. Both tests are positive in about 85 percent of patients. The indirect haemagglutination test is, however, more specific for this hydatid cysts.8
Complete removal of the cyst and prevention of recurrence with minimum morbidity and mortality are required to treat this disease. This can be done through different therapies such as systemic chemotherapy, laparoscopic interventions, and surgery.14 In most conservative surgical operations, the cyst cavity is opened with no peritoneal spillage. Then, a scolicidal agent is injected, which inactivates the protoscolices. Subsequently, the contents of the cyst are drained and the residual cavity is handled using different methods such as external drainage, omentopexy, capsulorrhaphy, capitonnage, and marsupialization.15
This paper reports the results of our surgical operations using the capsulorrhaphy method on 250 operations (130 men and 120 women), from 1989 to 2011.
Material and MethodsThis study, covering the years from 1989 to 2011, initially consisted of 282 patients with liver hydatid cysts. Thirty-two patients were eliminated from this study because they had cysts with infections, rupture, biliary contaminations, or small cysts in the center of the right lobe. The capsulorrhaphy technique was unsuitable for these kinds of cysts because of possible formation of an abscess after surgery. The studied 250 patients, who had no past medical history of liver disease, were admitted electively and underwent operations in Emam Khomeini, Emam Reza and other private hospitals in Tabriz. Medical information about patients such as age, sex, and hospitalizations, as well as the data about the cysts such as size, number, site, and type (intact or complicated) were collected and analyzed. Postoperative complications were assessed according to Clavien-Dindo classifications.16
The capsulorrhaphy technique was performed to remove the cysts. An incision was made along either of the midline or the right subcostal, depending on the number and location of cysts. After entering the peritoneal cavity, the area around the liver and the cysts was packed with swabs soaked with cetrimide 0.5%. The cyst was then punctured and its contents were aspirated using a large gauge needle. Aspiration of colorless and transparent liquid indicated an intact cyst. Powerful suction was placed at the base of the needle to prevent any liquid leakage and to avoid abdominal infection. After decreasing the cyst’s pressure equivalent of aspirated fluid, cetrimide 0.5% was injected into the cyst as a scolicidal agent and repeated after 5 min. Leakage of fluid was further prevented by packing the area around the liver with gauzes soaked with cetrimide, and by suctioning and pouring cetrimide 0.5% around the liver and onto the gauzes.
After decompressing the cyst contents using a needle, the cyst was taken with chromic sutures and was pulled up. The puncture was then enlarged and the contents of the cyst were extracted with suitable forceps. The inside of the cyst was reexamined until no effect of layers remained. Meanwhile, leakage of the fluid to the surrounding area was prevented by applying powerful suction. Any open bile ducts were closed with chromic sutures.
After complete evacuation and final cleaning of the cyst interior with swabs soaked in cetrimide 0.5%, the remaining cavity was either filled with normal saline and the pericyst layer cyst was reconstructed in two layers using zero chromic sutures, or the pericyst layer was first reconstructed and normal saline was then injected into it. Subsequently, the gauzes surrounding the cyst were removed and, after washing the surgery field with warm serum and counting the gauzes and surgical tools, two penrose drains were placed around the liver and then were brought out with a separate stab wound. Finally, the abdominal layer was sutured to the correct anatomical position.
Follow-ups included examinations for possible postoperative complications, such as infection, every week in the first month. Patients were informed about the symptoms of possible post-operative cyst recurrence, such as:
- •
Abdominal pain especially in right upper quadrant (RUQ).
- •
Feeling of a mass in the abdomen or in the RUQ.
- •
Icterus, and
- •
Productive cough.
And were asked to return to the hospital if they presented with any of them. Sonography was also performed between the third and sixth months after surgery to further examine the recurrence of hydatid cyst.
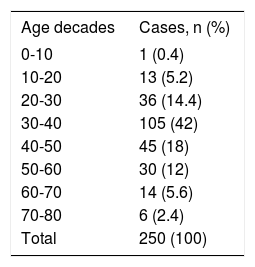
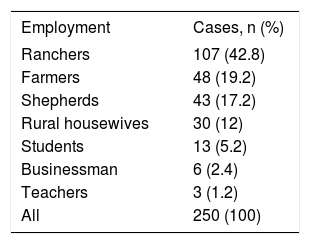
ResultsThe age of patients ranged from 9 to 80 years, with most in their forties. Table 1 shows the distribution of cysts by age. Patients held a variety of jobs but most were ranchers, farmers, shepherds, and rural housewives (Table 2). Making them more vulnerable to acquiring the disease from daily interaction with dogs and sheep.
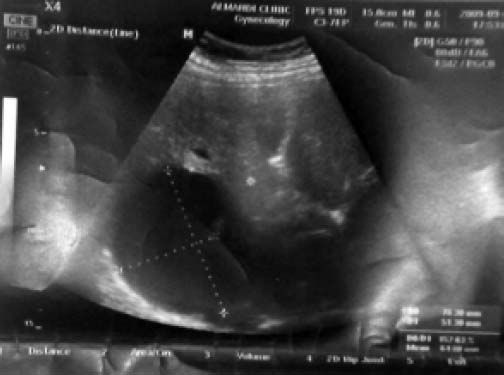
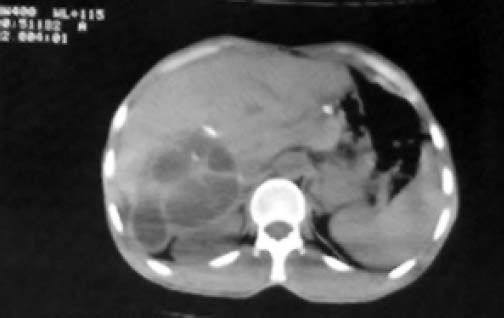
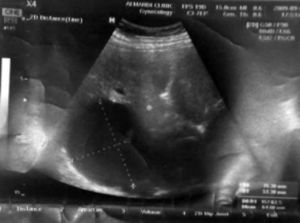
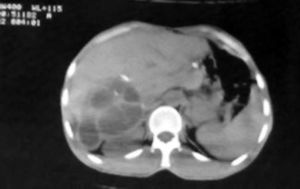
Among the studied patients, 206 (82.4%) of them were living in rural areas, 11 (4.4%) in urban areas and 33 (13.2%) frequenting both. Sonography was performed on all patients. The cyst diameter varied from 5 to 21 cm, most frequently between 10 and 20 cm. The sizes of all cysts are presented in table 3. Table 4 shows the location of these cysts in the liver. The capsulorrhaphy technique was used for type I, II, and III Gharbi classification regardless of cyst size. 40.8% of the patients were diagnosed with type II cyst. The frequency of cyst types III and I among the patients was 32% and 27.2%, respectively. Figure 1 shows a sonographic image of type II cyst according to Gharbi classification. In 8% of patients, a CT scan was used to confirm the diagnosis. Figure 2 shows a CT scan of a type III hydatid cyst.
The range of hospital stay was between 4 and 10 days, with an average of 5 days. Amongst the patients, 233 (93.2%) were discharged from hospital without any complications and 9 (3.6%) of them had wound infections in the abdominal wall. Seven (2.8%) of these patients had developed pulmonary atelectasis. The patients with abdominal wall infections and atelectasis were considered as a grade II complication according to Clavien-dindo classification. They stayed in the hospital for a few more days, and were treated with conservative therapy. One of the patients (0.4%) had external biliary fistula and bile leak, graded as a III-b complication. Accordingly, this patient underwent surgery and Roux-en-Y cystojejunostomy.
Patient follow-ups were held 1 to 24 months (mean 13.2 ± 8.5 months) after surgery. During this period, incisional hernias occurred in 5 (2%) patients. Hydatid cyst recurrence happened in 7 (2.8%) patients. The mortality rate was zero among the studied patients.
DiscussionA liver hydatid cyst is caused by the larval stage of Echinococcus granulosus,15 when a human becomes an accidental intermediate host for this larva, which lives as an adult worm in the canine intestine.8 Surgery for this disease is the cornerstone treatment. During open surgeries, the macroscopic parasite are eliminated, intraoperative spillage is inhibited and the residual cavity is obliterated if it is not resected.17
Open surgeries are classified into two categories: radical and conservative. The radical procedures are pericystectomy or hepatic resection. The conservative techniques deroof the cysts and manage the residual cavity18 by external drainage, omentopexy, capsulorrhaphy, capitonnage and marsupialization.15 Conservative methods are safer, technically simpler and more useful in the management of uncomplicated hydatid cysts compared to other techniques. Their postoperative complications are their main disadvantage.19 We report 0.4% biliary complications, while the occurrence of biliary complications after surgery was reported to be 16% by Agarwal, et al.,20 similar to those in other series with predominantly conservative surgical techniques of management.19,21–24 The difference shows that the capsulorrhaphy technique has a lower rate of biliary complications than other conservative techniques. It should be noted that we have used this technique in patients with no complications at the first diagnosis and this might have contributed to the lower rate of biliary complications.
The results reported by Mousavi, et al. on 65 patients indicate that the postoperative wound infection rate after omentoplasty was 5.7% and after simple drainage it was 13.3%.4 Avgerinos, et al. reported 6% post operative wound infection, after surgery on 35 patients.25 Compared to these studies, wound infection after capsulorrhaphy method was lower (3.6%), as reported in our study. Avgerinos, et al. reported 3% suppuration and abscess formation in the residual cavity,25 whereas we report no abscess formation after surgery. Busuioc, et al. reported the rate of postoperative incisional hernia for uncomplicated cysts to be 1.2%,26 but our study found the rate to be at 2%. This difference is perhaps due to different surgical technique used in that study.
One of the important problems in the management of liver hydatid cysts is the recurrence of the cyst in about 1.1-25 percent of cases.23,27–35 Recurrence of the hydatid cyst occurs as a new cyst inside or outside the liver. In our research, the recurrence rate was approximately 2.8%. The low recurrence rate reported in our study was perhaps due to our careful suction and prevention of the spillage of the cyst contents. The rate might be underestimated because some patients might have returned to different hospitals during long-term follow-ups.
Postoperative complications increase if the patients are followed-up with other physicians. Often, there is a chance that capsulorrhaphy treated locations are mistakenly identified as a new cyst, the remainder of a cyst or an abscess in a sonographic examination after a short postoperative period up to several months. To avoid misdiagnosis, it is important to tell the patients to inform their future physicians about their history of capsulorrhaphy operation.
Our study reports no mortality; however, Monro, et al. reported a high mortality rate, about 27-32.5% after using surgical treatments.22 Zaouche, et al. reported a mortality rate of about 4.5% in their study of 244 patients who underwent surgical operation for hepatic cysts of liver with a large biliocystic fistula.27 In another study, the mortality rate in patients who underwent conservative and radical surgery was reported at 5.9 and 1.8%, respectively.28 Some studies have reported a low mortality rate of about 0-3% after hydatid cyst treatment.23,29,36 Gokhan, et al. reported a 1.08% mortality for the patients with open surgery operations.14 Seven, et al.37 and Alper, et al.38 reported zero mortality rate for laparoscopic surgery. The difference in mortality rate in these studies indicates that it depends on the cases, the condition of surgery, preoperative and postoperative complications.
Another benefit of capsulorrhaphy is short length of hospitalization. According to our research, the mean time of hospitalization was 5 days, whereas it was 7 days for those without biliary compilations,20 15.6 days for conservative method with omentoplasty, and 6.5 days for drainage techniques.4
ConclusionCapsulorrhaphy is an efficient and safe technique to eradicate residual cavities of liver hydatid cysts and to reduce the postoperative complications, recurrence, and hospitalization period. Capsulorrhaphy has a low morbidity and mortality rate in comparison with other techniques and can be considered as the method of choice in the management of uncomplicated hydatic cysts.
AcknowledgmentThe authors wish to thank Dr. Hossein K. Heris (McGill University) and Mr. Steven Reiter (McGill University) for proofreading this manuscript.
Source of FundingNone.