Non-selective beta blockers are very useful drugs in preventing first variceal bleeding and re-bleeding in patients with cirrhosis. These drugs work in two ways: 1) by blocking β1 receptors and reducing cardiac output, and 2) by blocking β2 receptors, producing splanchnic vasoconstriction and reducing portal flow. Consequently, they reduce portal pressure. In primary prophylaxis, beta blockers reduced the bleeding risk from 30 to 15%; in secondary prophylaxis, this risk decreased from 60 to 42% in the first year. Heart rate decrease does not necessary correlate with reduction in hepatic venous pressure gradient (HVPG). When this gradient is reduced to less than 12 mmHg, the patient will not bleed; when this is reduced > 20% from basal values bleeding risk is extremely low, estimated at 9% at 2 years. The only way to know whether the patient has become a responder is to measure the HVPG. Additionally, by means of this method we also can identify the non-responders, who have a higher rate of re-bleeding, between 54 and 64%, and can attempt to utilize a more aggressive therapy, such as adding isosorbide mononitrate to the beta blocker or combining the beta blocker with endoscopic ligation. These options are discussed in the present review.
In the physiopathology of portal hypertension, there is an increase in portal blood flow due to splanchnic vasodilatation. Moreover, there is intrahepatic resistance to this blood flow, the first known mechanism of portal hypertension. This is due not only to an alteration in hepatic architecture, but also to a dynamic situation originating in the contraction of perivascular smooth muscle cells, myofibroblasts, and hepatic stellate cells, which represent approximately 30% of global intrahepatic resistance, this concept first described by Bathal and Groszmann.1 The previously mentioned mechanisms increase portal territory pressure. By employing hepatic vein pressure gradient (HVPG = wedged hepatic pressure-free hepatic pressure) as a portal pressure reflex, it is known that an HVPG of >10 mmHg is required for esophageal varices to appear, and an HVPG of over 12 mmHg is a requirement for these varices to bleed.2
More than 40% of patients with cirrhosis have esophageal varices at the moment of diagnosis. Approximately 30% of these patients with large varices will experience a bleeding episode in the subsequent 2 years,3 with a 1-year re-bleeding possibility of approximately 60% and mortality of 20% in each episode.4 We will describe the usefulness of non-selective beta blockers in primary and secondary prophylaxis.
Primary prophylaxis (Prevention of the first bleeding episode)Beta blockersIn 1981, Lebrec published the use of propranolol in prevention of variceal re-bleeding, and in 1987 Pascal employed propranolol as primary prophylaxis. Propranolol and nadolol, which are non-selective beta blockers, reduce portal pressure via two mechanisms: 1) Cardiac output is reduced by blocking β1 adrenergic receptors, 2) Splachnic vasoconstriction by blocking β2 receptors (vasodilators). By means of these two mechanisms splanchnic flow is reduced, as is portal pressure, reflecting a reduction of pressure in collateral veins (varices) and also in their walls.
The usefulness of beta blockers has been evaluated and compared against placebo in 12 randomized trials. Several meta-analyses show a reduction in bleeding risk, demonstrating that non-selective beta blockers constitute an efficacious therapy in primary prophylaxis. Patients with large varices had a 30% risk of first bleeding in the following 24 months; beta blockers reduced this risk to 15%.5,6 This means that beta blocker utilization allows a global reduction of 50% in risk of first variceal bleeding episode. It is clear that non-selective beta blockers do not protect all patients, because there remains a 15% bleeding risk in the subsequent 2 years in patients using beta blockers; nonetheless, this fact might be due to insufficient reduction in HVPG and to differences in individual sensitivity to beta blockers depending on age, weight, genetic polymorphisms of beta adrenoreceptors, and amount of portosystemic collaterals. According to these facts, treatment of 11 patients is required to prevent one variceal bleeding episode.
Previous reports demonstrated that reducing HVPG to less than 12 mmHg nearly totally lowers risk of bleeding.2,7 Other studies have shown that even without reaching these values, reducing HVPG by at least 20% of the basal value is related with lower bleeding risk, estimated at between 4 and 9% in 1 and 2 years, respectively.8 Also, it has been demonstrated that patients reaching the previously mentioned hemodynamic goals exhibit a marked reduction in the risk of developing other complications of portal hypertension such as ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy, in addition to having improvement in survival rates when compared with non-responders.9
Once the hemodynamic goal is reached, it is sustained in the majority of patients. Villanueva et al.10 studied 64 patients with sustained hemodynamic response and measured HVPG during the first three months of treatment and also at 18 months of treatment. They showed that 81% of these patients maintained hemodynamic response.
Beta blocker plus isosorbide mononitrateOne third of patients under beta blocker treatment achieve a reduction of ≥ 20% of basal HVPG or less than 12 mmHg. There are other treatments that have been tested for increasing this response. In different trials,11,12 it was observed that a combination of a beta blocker plus isosorbide mononitrate increases hemodynamic response in 30% of patients who were non-responders with a beta blocker alone. This is due to the increase in intrahepatic nitric oxide, which reduces resistance to the portal flow by relaxing intrahepatic vascular tone. Merkel13 suggests that combined treatment is superior to beta blockers alone in primary prophylaxis, showing a marked reduction of first bleeding episode with a p value of 0.02 in 84 months of follow up, although without a survival difference. Nevertheless, another prospective and multicenter trial with 349 patients randomized to propranolol vs pro-pranolol plus isosorbide mononitrate with unknown hemodynamic response to beta blocker alone demonstrated no benefit with propranolol plus isosorbide mononitrate in primary prophylaxis; bleeding risk in the first group was 10.6 against 12.5% in the second group.14 At present, it is considered that there is not sufficient evidence for use of the combined treatment as primary prophylaxis.
Hemodynamic response monitoringHemodynamic response is achieved independently from lowering heart rate to fewer than 55 beats per minute.15 This means that monitoring heart rate does not allow identifying responders. It is useful to dosify the medication, but this does not correlate with lowering the HVPG. There have been attempts to monitor hemodynamic response by other means, such as Doppler ultrasound, but this has shown no correlation with HVPG; in addition, intravariceal pressure measurement is an invasive method with little reproducibility. Measuring hepatic pressure should be practiced on each patient included in clinical trials because effective treatments must be shown in non-responders. In addition to clinical trials, cost analyses are inconclusive and hepatic catheterization should depend on the hospital, patients, healthcare specialists, and health policies involved. From a practical point of view, 60% of patients receiving beta blockers as primary prophylaxis not achieving the previously mentioned goals will not bleed in the following 24 months.5 This renders it unnecessary to measure HVPG in primary prophylaxis; notwithstanding this, in secondary prophylaxis with high risk of re-bleeding it would be optimal to measure HVPG in each patient.
Endoscopic treatmentIn primary prophylaxis, endoscopic sclerotherapy is not recommended due to the morbidity related with this procedure. A meta-analysis comparing endoscopic variceal ligation (EVL) vs beta blocker treatment16 estimated an odds ratio (OR) for first bleeding episode of 0.48 (0.24 to 0.96), with a necessary number to treat (NNT) of 13 patients in favor of EVL, although it does not offer great advantages with regard to survival. Thus, EVL is not recommended as primary prophylaxis17 at present. EVL should be considered for the patient who is unable to tolerate or does not respond to beta blocker therapy. Additionally, this meta-analysis has been criticized for including trials published only as summaries and also for including one trial in which number of patients who bled under beta blocker treatment was higher than in other reports; as if this were not sufficient, no cost analyses were included for EVL. In a new trial18 in which 50 patients were randomized for banding and 50 for beta blocker therapy as primary prophylaxis, there was a bleeding frequency at 22 months of 10 patients (20%) for the first group and 16 patients (32%) for the second group (p = 0.23) without a difference in mortality. Future trials should focus on comparing EVL and beta blocker therapy with respect to survival and cost analysis. Preliminary data suggest that propranolol plus EVL is not better than banding alone in primary prophylaxis,19 although it does reduce variceal recurrence. The same study published in an extended form20 reported that both endoscopic banding plus propranolol and endoscopic banding alone are effective in primary prophylaxis of bleeding from high-risk varices. Addition of propranolol does not decrease the probability of first bleeding or death in patients on EVL; however, varices recurrence is lower if propranolol is added to EVL.
Secondary prophylaxis (Re-bleeding prevention)Patients who survive the esophageal varices-related first bleeding episode have a high re-bleeding risk, nearly 60% in 1 year.4
Beta blockersAs in primary prophylaxis, employment of non-selective beta blockers reduces portal pressure. Different meta-analyses have shown that use of these drugs reduces the re-bleeding risk in 1 year from 60 to 42%.6 Patients who benefit in these trials are those achieving a high hemodynamic response. Next, we will clarify the usefulness of beta blocker plus isosorbide mononitrate and beta blocker plus endoscopic banding.
Beta blocker plus isosorbide mononitrateA controlled trial comparing combined treatment vs. monotherapy showed the combination to be better. Although there was no significant difference at the beginning, nonetheless when the authors stratified by age the difference appeared in patients under 50 years of age and in those with 1 additional year of follow-up.21 Bureau et al22 identified patients who did not respond to propranolol by practicing hemodynamic studies on these. Next, they administered isosorbide mononitrate to them and achieved to increase responder number from 38 (13 patients) to 59% (20 patients) in a total of 34 patients. The conclusion of this trial comprises that hemodynamic studies allow to identify patients who will benefit from combined treatment. HVPG measured in the first 2 treatment weeks is required in all patients for this purpose. Some criticism generated by this trial includes the fact that propranolol was used at a fixed dose of 160 mg and that the drug was obtained from a long-acting preparation. We do not know whether a higher dose could have increased response to beta blocker alone because the recommendation is to administer the highest tolerated dose. What is important in identifying non-responders is that treatment can be modified to achieve an HVPG reduction. Patients responding to beta blocker alone, those already achieving hemodynamic response, derive no benefit from addition nitrates to their treatment.
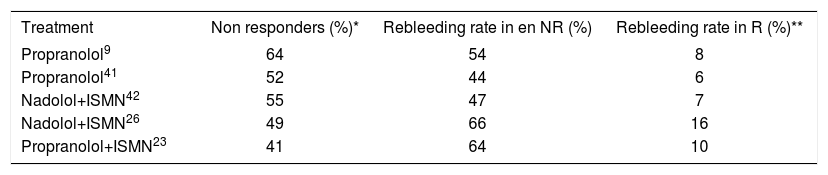
Beta blocker plus endoscopic bandingNevertheless, there is an important group of non-responding patients with a high re-bleeding risk, as shown in Table I. Percentage of re-bleeding in non-responding patients to propranolol alone and to the combination of propranolol and isosorbide mononitrate is observed there. Percentage of re-bleeding is less in patients achieving hemodynamic response. The best treatment option for patients in whom combination therapy is not effective remains unknown to date. One option for these patients is to utilize endoscopic banding; however, in Bureau’s trial22 banding was practiced in eight non-responders, and seven re-bled. Although it appears that esophageal varices were not treated totally, this finding is very important because this is the first trial to report the usefulness of banding in patients not responding to combined treatment with beta blockers and nitrates, this not very encouraging. On the other hand, Lo23 reported a low frequency of re-bleeding with combining banding and beta blocker vs. banding alone (23 vs 47%, respectively; p = 0.005). Similar results were showed by De la Peña et al,24 suggesting that the combination of banding and beta blocker can be a useful alternative. However, these are the only controlled trials; thus, additional information is needed to confirm these results.
Beta blocker vs endoscopic bandingBaveno’s consensus highlighted endoscopic banding as a first-line treatment, similar to beta blocker therapy in secondary prophylaxis. The following question must be posed: Which of the two treatments should be used? When there is a contraindication for use of beta blocker the answer is obvious, but when there is no contraindication, there are no clear statements to rely on for decision-making. There are three trials that compare pharmacologic treatment (beta blocker plus isosorbide mononitrate) vs banding. One shows more benefit from the pharmacologic therapy,25 the second shows an identical benefit from both treatments,26 and the third one places endoscopic banding over pharmacologic therapy,27 without a difference in mortality in any of these. On the other hand, older trials that compared re-bleeding frequency between both treatments found that banding possessed a re-bleeding frequency of 16 to 29%; more recent trials found a higher re-bleeding frequency (38 to 56%).28 This finally states that endoscopic banding is not better than beta blocker treatment. In addition, banding acts locally in varices without improving portal hypertension physiopathology. Endoscopic banding is effective, but for a short time only because portal pressure and flow are not modified and there is a recurrence of varices up to 50% in 2 years. This renders endoscopic follow-up necessary.29
Derivative surgeryDerivative surgery has been used for over 50 years in secondary prophylaxis. It is based on shunting the blood flow from the portal territory. It is a useful alternative for reducing re-bleeding risk, but with the disadvantage of increasing encephalopathy and hepatic failure in non-selective techniques. There are efficient selective proceedings to reduce adverse effects, such splenorrenal shunts or small-diameter mesocaval techniques utilizing grafts, although these proceedings require patients with low surgical risk and there is little experience in controlled trials.
Transjugular intrahepatic portosystemic shunts (TIPS)TIPS result in very controlled shunting with respect to diameter. Additionally, a surgical procedure is not necessary; thus, related morbimortality is very low. Nevertheless, results are not encouraging due to the dysfunction rate of more than 50% in the first year due to proliferation of the intimae into the shunt. This increases the need for angiographic surveillance to assess TIPS permeability and also increases the encephalopathy rate from 20 to 30%. In Escorcel’s trial,30 the authors demonstrated a marked reduction in re-bleeding on comparing TIPS vs. endoscopic/pharmacologic treatment; however, the trial also showed that costs doubled without a real benefit with respect to survival and quality of life. A recent trial suggests that utilizing polytetrafluoroethylene-covered TIPS is associated with longer shunt permeability without increasing the encephalopathy rate.31 At present, surgery and TIPS are only recommended as rescue therapies in patients with failure in endoscopic or pharmacologic treatments.
Bosch and Garci’a-Pagan28 suggested a practical approach to secondary prophylaxis: initiation of treatment with a beta blocker, except in patients unable to tolerate this or in patients with a contraindication, and addition of endoscopic banding in patients who bled while on a beta blocker (including patients who had their first bleeding episode while on a prophylactic beta blocker). Isosorbide mononitrate can be added to all patients, especially if HVPG measurement is not performed, or it can be added selectively to those patients not responding to beta blocker alone, identified by measuring basal and post treatment HVPG.
Some practical issues in the use of these drugs are mentioned next. Propranolol is administered orally twice a day, increasing the dose every 2 days so that the titering phase is as short as possible, ideally 2 weeks. The goal is to achieve a 25% reduction in basal heart rate or to reach 55 beats per minute. If nadolol is employed, it is recommended that this be administered once a day because of its longer half-life. Doses are different in each trial, but in general it is accepted that higher doses reach the hemodynamic target. In the Abraldes’ trial9 that demonstrated better survival in hemodynamic responders, mean propranolol doses were higher in responders than in non-responders (150 ± 97 mg/d vs. 89 ± 56 mg/d, respectively; p = 0.002).
Prior to initiating beta blocker therapy, the patient should have an electrocardiogram to ensure that a blockage does not exist and also to rule out asthma or hypoglycemia. When the drug therapy for use is the beta blocker plus isosorbide mononitrate combination, the maximum tolerated dose of the first drug should be achieved; only after that should the latter be initiated. There is no difference between propranolol and nadolol in portal pressure control.3 Intolerance to one of these drugs can be solved by changing it for the other.32 Once a beta blocker is initiated, it should be continued indefinitely because suspension is related with an increase in bleeding risk that equals this risk in untreated individuals.33
Carvedilol is a non-selective beta blocker and a1 adrenergic antagonist, and has also been studied for treatment of portal hypertension. Acute carvedilol administration induces higher reduction in portal pressure than propranolol, with lowering of arterial pressure and vascular resistance;34 this can have a harmful effect on renal function. Further trials were carried out with long-term carvedilol35 and results were the same; therefore, its clinical application is limited.
The role beta blockers play in retarding variceal progression has not been established. Animal trials have shown reduction in collateral formation.36,37 A human trial38 demonstrated no benefit from propranolol in delaying variceal progression, although a recent trial39 showed that nadolol had a positive effect. This suggests that nadolol should be started when small varices are detected, but this behavior must to be proven by additional trials.
Finally, in relation to gastric varices (GV) these represent 5 to 10% of upper gastrointestinal tract bleeding cases in patients with cirrhosis. Treatment for GV bleeding has not been evaluated in controlled clinical trials, and the majority of available information derives from retrospective trials and case reports. Due to the fact that risk of bleeding from GV is lower than from esophageal varices, use of beta blockers for primary prophylaxis in GV40 is suggested.