Background. Hepatopulmonary syndrome (HPS) is a complication of advanced liver disease. The impact of HPS on survival is not clearly understood.
Material and methods. A prospective study was carried out at Department of Medicine, King Edward Medical University Lahore from June 2011 to May 2012. Patients with cirrhosis of liver were evaluated for presence of HPS with arterial blood gas analysis and saline bubble echocardiography. All patients were followed for 6 months for complications and mortality. Cox regression analysis was done to evaluate role of HPS on patient survival.
Results. 110 patients were included in the study. Twenty-nine patients (26%) had HPS. MELD score was significantly higher (p < 0.01) in patients with HPS (18.93 ± 3.51) as compared to that in patients without HPS (13.52 ± 3.3). Twenty two (75.9%) patients of Child class C, 5 (17.2%) patients of Child class B and 2 (6.9%) patients of Child class A had HPS (P 0.03). The clinical variables associated with presence of HPS were spider nevi, digital clubbing, dyspnea, and platypnea. HPS significantly increased mortality during six month follow up period (HR: 2.47, 95% CI: 1.10-5.55). Child-Pugh and MELD scores were also associated with increased mortality. HPS was no longer associated with mortality when adjustment was done for age, gender, Child-Pugh, and MELD scores (HR: 0.44, 95% CI: 0.14-1.41). Both the Child-Pugh and MELD scores remained significantly associated with mortality in the multivariate survival analysis.
Conclusions. HPS indicates advanced liver disease. HPS does not affect mortality when adjusted for severity of cirrhosis.
Hepatopulmonary syndrome (HPS) consists of triad of liver disease, systemic hypoxemia, and pulmonary vasodilatation. It occurs in 10-32% of patients with cirrhosis.1 Although it is commonly seen in advanced cirrhosis, however it has also been observed in acute hepatitis and non-cirrhotic portal hypertension.2,3 Hepatopulmonary syndrome is usually a feature of advanced liver disease and should be considered in any patient who presents with liver disease and hypoxia. Liver transplant is the only definitive treatment for HPS.
The pathogenesis of HPS is not clearly understood but pre-capillary dilatation in lungs play a central part. It leads to shunting of blood within pulmonary vasculature, ventilation-perfusion mismatch, and increased alveolar-arterial oxygen saturation gradient.1 Ventilation-perfusion mismatch results in increased blood flow in areas of lung that are less ventilated and so leads to decreased oxygenation of blood. Dyspnoea is very common in HPS. More specific symptom is platypnea, which is shortness of breath when patient sits up from supine position. It happens because of orthodeoxia, which is drop in oxygen saturation of blood from supine to sitting posture.4 Nitric oxide (NO) is mainly implicated in causing pulmonary pre-capillary vasodilatation. It has been observed that level of NO in exhaled air is higher in cirrhotic patients with HPS than those in cirrhotic patients without HPS.5
HPS affects quality of life. In one study it was observed that HPS was associated with poor quality of life in cirrhotic patients who were waiting for liver transplant.6 Although, HPS is associated with adverse clinical outcomes, its effect on mortality is not clearly understood. Moreover no study in Pakistan has prospectively evaluated the impact of HPS on patient survival. The aim of our present study was to assess the prevalence and predictors of HPS and prospectively evaluate the effect of HPS on mortality in patients with advanced cirrhosis.
Material and MethodsThis study was carried out at Department of Medicine King Edward Medical University from June 2011 to May 2012. A total of 110 consecutive patients of previously diagnosed chronic liver disease were included in the study. Patients were recruited both from out-patient and in-patient departments. Patients of chronic renal failure, left heart failure, valvular heart disease, pulmonary tuberculosis, chronic obstructive airway disease, asthma or interstitial lung disease and diagnosed malignancy were excluded. Patients with congenital heart diseases, smokers and those with hemoglobin less than 10 g/ dL were also excluded. A detailed clinical history was taken and complete physical examination was performed. Signs that were specifically looked for included digital clubbing, palmer erythema, jaundice, gynecomastia, spider nevi, and caput medusa.
Liver function tests, serum albumin, prothombrin time, viral markers, complete blood count with platelets, blood urea nitrogen and serum creatinine were measured. X-ray chest, ECG and echocardiography were done for all patients to rule out cardiac or respiratory problem. Ultrasound abdomen was done to assess liver texture, liver, spleen, and portal vein diameters, and presence of ascites. Liver disease severity was assesses by calculating Child-Pugh and Model for Endstage Liver Disease (MELD) scores.
Assessment of HPS was undertaken after initial clinical improvement after taking written informed consent. One sample of blood was obtained by percutaneous radial artery puncture in a stable seated patient while breathing room air and ABGs analysis done by using Arterial Blood Gases Analyzer (Rapid Lab 348). Hypoxemia was defined as PO2 of less than 80 mmHg. Oxygen saturation by digital pulse oximeter was noted both in supine and erect position.
Contrast enhanced echocardiography was done on echocardiography machine (GE-LOGIC 5 PRO) by one of the investigators. The presence of pulmonary vascular dilatation was indirectly determined by qualitative assessment of right to left shunting using saline micro-bubble injection along with transthoracic contrast enhanced echocardiography. For this test 10 ml of agitated saline was injected via right anti-cubital vein. Those with appearance of bubbles in left atrium after 3 to 7 heart beats, in the absence of intra-cardiac communications, were considered as evidence of pulmonary vascular dilatation. Patients were kept in ICU after the procedure and monitored for adverse events.
Diagnosis of HPS was based on hypoxemia and demonstration of intrapulmonary shunting in patients with cirrhosis. Two close contact persons were identified for each patient and after the patient was discharged, monthly follow up was done through telephone calls for 6 months. Information was obtained regarding clinical condition of the patient, new hospital admissions and complications, and whether the patient was alive or not.
Data analysisStatistical Program for Social Sciences v20 and STATA v12 were used for data analysis. Quantitative data was summarized as mean ± standard deviation. Qualitative data was summarized as percentages and frequencies. Patients with and without HPS were compared using Chi square test for categorical variables and unpaired two tailed students (t) test for quantitative variables. Survival analysis was carried out through Cox-proportional hazard regression to evaluate variables affecting patient survival. Failure event was defined as death during follow up. Log-log plots were analyzed to check whether model assumptions were met or not. Unadjusted hazard ratios with corresponding 95% confidence intervals were calculated for each independent variable. In the multivariate Cox regression model, hazard ratio for HPS was adjusted for age, sex, MELD and Child-Pugh scores. P value < 0.05 was considered significant for all analyses.
The study was approved by the institutional review board of King Edward Medical University and Mayo Hospital.
ResultsOne hundred and ten patients of cirrhosis were included in the study. Male to female ratio was 2.3:1 (77/33) and mean age of whole sample was 56.15 ± 9.68 years.
Etiology of cirrhosis was hepatitis C virus infection in 77(70%) patients, hepatitis B infection in 10(9.1%) patients, both hepatitis B and Cin 8 (7.3%), alcoholic liver disease in 7(6.4%) patients. Five patients (4.5%) were negative for both hepatitis B and C; two patients had drug-induced liver cirrhosis; and one was diagnosed with Wilson’s disease. Presenting complaint was upper gastrointestinal bleeding (UGIB) in 32(29.1%) patients, spontaneous bacterial peritonitis and urinary tract infection in 24 (21.8%) patients, and hepatic encephalopathy in 27 (24.5%). Ascites was present in 90(81.8%) patients.
Sixty six (60%) patients were of Child Pugh Class C, 25 (23%) were of class B and 19 (17%) of class A.
Hypoxemia (PaO2 less than 80 mmHg) was observed in 30 (27%) patients and the presence of pulmonary vascular dilation was observed in 33 (30%) patients. Twenty nine patients (26%) had both hypoxemia and evidence of pulmonary vascular dilatation on echocardiography and thus were diagnosed with HPS.
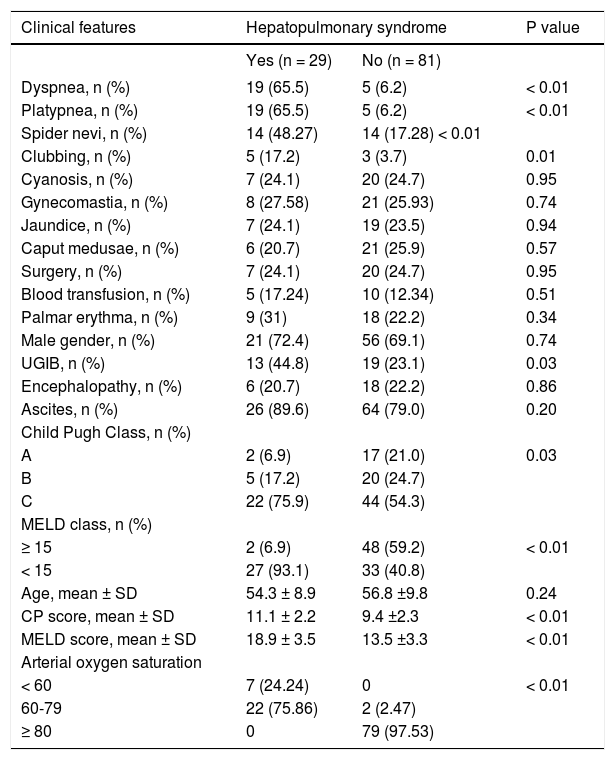
We compared patients with and without HPS as shown in table 1. Patients with and without HPS did not differ regarding age and sex. The clinical variables significantly associated with HPS were presence of spider nevi (48.25% of patients with HPS vs. 17.28% of patients without HPS, P < 0.01) and digital clubbing (17.2% of the patients with HPS vs. 3.7% of the patients without HPS, P = 0.01). Dyspnoea and platypnea were also significantly associated with HPS.
Comparison of patients with and without hepatopulmonary syndrome.
| Clinical features | Hepatopulmonary syndrome | P value | |
|---|---|---|---|
| Yes (n = 29) | No (n = 81) | ||
| Dyspnea, n (%) | 19 (65.5) | 5 (6.2) | < 0.01 |
| Platypnea, n (%) | 19 (65.5) | 5 (6.2) | < 0.01 |
| Spider nevi, n (%) | 14 (48.27) | 14 (17.28) < 0.01 | |
| Clubbing, n (%) | 5 (17.2) | 3 (3.7) | 0.01 |
| Cyanosis, n (%) | 7 (24.1) | 20 (24.7) | 0.95 |
| Gynecomastia, n (%) | 8 (27.58) | 21 (25.93) | 0.74 |
| Jaundice, n (%) | 7 (24.1) | 19 (23.5) | 0.94 |
| Caput medusae, n (%) | 6 (20.7) | 21 (25.9) | 0.57 |
| Surgery, n (%) | 7 (24.1) | 20 (24.7) | 0.95 |
| Blood transfusion, n (%) | 5 (17.24) | 10 (12.34) | 0.51 |
| Palmar erythma, n (%) | 9 (31) | 18 (22.2) | 0.34 |
| Male gender, n (%) | 21 (72.4) | 56 (69.1) | 0.74 |
| UGIB, n (%) | 13 (44.8) | 19 (23.1) | 0.03 |
| Encephalopathy, n (%) | 6 (20.7) | 18 (22.2) | 0.86 |
| Ascites, n (%) | 26 (89.6) | 64 (79.0) | 0.20 |
| Child Pugh Class, n (%) | |||
| A | 2 (6.9) | 17 (21.0) | 0.03 |
| B | 5 (17.2) | 20 (24.7) | |
| C | 22 (75.9) | 44 (54.3) | |
| MELD class, n (%) | |||
| ≥ 15 | 2 (6.9) | 48 (59.2) | < 0.01 |
| < 15 | 27 (93.1) | 33 (40.8) | |
| Age, mean ± SD | 54.3 ± 8.9 | 56.8 ±9.8 | 0.24 |
| CP score, mean ± SD | 11.1 ± 2.2 | 9.4 ±2.3 | < 0.01 |
| MELD score, mean ± SD | 18.9 ± 3.5 | 13.5 ±3.3 | < 0.01 |
| Arterial oxygen saturation | |||
| < 60 | 7 (24.24) | 0 | < 0.01 |
| 60-79 | 22 (75.86) | 2 (2.47) | |
| ≥ 80 | 0 | 79 (97.53) | |
Patients with HPS had significantly higher MELD scores when compared with patients without HPS (18.93 ± 3.51 vs. 13.52 ± 3.33, P ≤ 0.01). Only 2 (6.9%) patients fell into MELD category I (< 15), while 27 (93.1%) patients came in category II (≥ 15) (P < 0.01). The Child-Pugh score was also higher in HPS patients (11.1 ± 2.2), than patients without HPS (9.4 ± 2.3) (P < 0.01). Out of 29 patients with HPS, 22 (75.9%) patients were of Child class C, 5 (17.2%) patients of Child class B and 2 (6.9) patients were child class A.
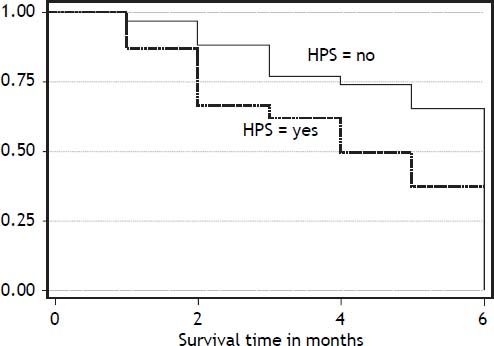
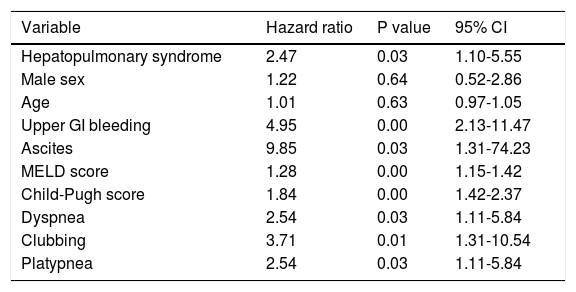
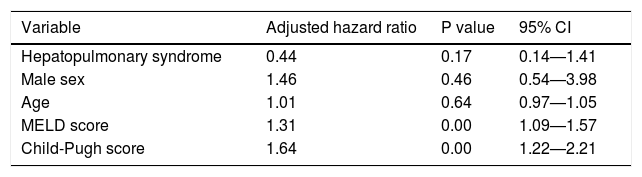
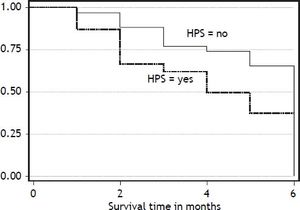
Six months post discharge follow up was possible in 59 patients. Twenty seven patients (45.7%) died during the follow up period. Out of 24 patients with HPS followed, fifteen (62.5%) died during next six months, while 12 of 35 patients (34.2%) without HPS died during this period. Figure 1 describes the Kaplan-Meier survival curves of patients with and without HPS. Table 2 shows unadjusted estimates of bivariate Cox regression analysis. Variables associated with increased mortality on bivariate Coxregression analysis were HPS, upper gastrointestinal bleeding, Child-Pugh and MELD scores, ascites, dyspnea, platypnea, and clubbing. Patients with HPS were 2.47 times more likely to die than patients without HPS during the follow up period. But HPS was no longer associated with increased mortality when adjustment was done for age, gender, Child-Pugh, and MELD scores (Table 3). In the adjusted model, MELD and Child-Pugh scores remained significantly associated with survival.
Unadjusted estimates of survival among patients with cirrhosis.
| Variable | Hazard ratio | P value | 95% CI |
|---|---|---|---|
| Hepatopulmonary syndrome | 2.47 | 0.03 | 1.10-5.55 |
| Male sex | 1.22 | 0.64 | 0.52-2.86 |
| Age | 1.01 | 0.63 | 0.97-1.05 |
| Upper GI bleeding | 4.95 | 0.00 | 2.13-11.47 |
| Ascites | 9.85 | 0.03 | 1.31-74.23 |
| MELD score | 1.28 | 0.00 | 1.15-1.42 |
| Child-Pugh score | 1.84 | 0.00 | 1.42-2.37 |
| Dyspnea | 2.54 | 0.03 | 1.11-5.84 |
| Clubbing | 3.71 | 0.01 | 1.31-10.54 |
| Platypnea | 2.54 | 0.03 | 1.11-5.84 |
This study demonstrated that 30% patients hospitalized for chronic liver disease had intrapulmonary vascular dilatation and 26% had hepatopulmonary syndrome. Hypoxemia was observed in 27% the patients. These data are in accordance with previous reports that have found the presence of intrapulmonary vascular dilatation in 34% and HPS in 10-32% of the patients1,7–9 using different cut-off levels of PaO2 and AaO2 for the definition of HPS.
As observed in some previous studies,10,11 patients with HPS in the present study had significantly more spider nevi and finger clubbing when compared to their counterparts without HPS. It has been noted previously that patients with cutaneous spider nevi have more profound gas exchange abnormalities and more intrapulmonary vasodilation.12,13 Spider nevi are suggested by some authors as cutaneous marker of intrapulmonary vascular dilatations.14 Our study supports these previous reports even with small sample. As expected, a high percentage of patients with HPS had dyspnea 19 (65.5%) while only 5 (6.2%) patients without HPS had this symptom.
Conflicting data exist in the literature regarding the correlation between HPS and the severity of liver disease. Many studies did not find association between HPS and Child-pugh score.10,11,15,16 In one study HPS was associated with higher Child-Pugh score but it did not have any relation with MELD score.17 In another study too, MELD score was not associated with HPS.6 Our study clearly showed a significant association between Child-Pugh and MELD scores. Similar association regarding presence of HPS and liver disease severity was found in many previous studies.10,13,18 Moreover Child-Pugh score has been associated with severity of HPS.13 In addition we also observed that HPS can occur in patients having low Child-Pugh or MELD score, but chances increases as severity of liver disease increases.
Recently Ferreire, et al.12 found hepatopulmonary syndrome in 16% of patients with chronic liver disease. Patients with HPS in that study had more severe liver disease assessed by the MELD score, but not by Child-Pugh classification. The difference may be due to selection of the patients. In that study all the patients were of decompensated CLD having high Child-Pugh scores while in our study both compensated and decompensated patients of CLD were included. They found no relation between HPS and dyspnea and platypnea.
So far no regional study has been done to see the correlation between severity of liver disease and occurrence of HPS; only frequency of HPS in cirrhotic patients was observed by Shafique, et al.19 In their study, 28.9% patients with cirrhosis of the liver had HPS. All belonged to child class C. Digital Clubbing, arterial hypoxemia and intrapulmonary vascular dilatations were important features of hepatopulmonary syndrome. All these features are much similar to our results. However, we found HPS in all three Child-Pugh classes as compared to their study in which all patients of HPS were in class C. This difference may be due to patients’ selection. They studied admitted patients only which were more likely to have advanced disease. However our sample comprised both admitted and out-patients which gave us the opportunity to observe HPS in whole spectrum of disease severity.
We found that HPS, Child-Pugh and MELD scores are associated with increased mortality in patients with cirrhosis. When adjustment was done for age, sex, Child-Pugh and MELD scores, HPS was no longer affecting mortality while Child-Pugh and MELD scores remained independent predictors of mortality in patients with cirrhosis. Since HPS is associated with Child-Pugh and MELD scores, as described above in our study, it seems that significant effect of HPS on mortality in unadjusted model is because of confounding by disease severity measured in terms of Child-Pugh and MELD scores. Similar results were found in one previous study in which HPS did not impact mortality in cirrhosis.20 In another study, HPS did not affect in-hospital mortality in patients with decompensated cirrhosis.17 Similar findings have been reported in a recent study by Pascasio, et al., who evaluated patients in liver transplant waiting list to study the effect of HPS on survival. They found that HPS did not affect survival before and after liver transplant.21 Another recent study using data submitted to the United Network for Organ Sharing found that transplant recipients with an baseline room-air PaO2 ≤ 44.0 mmHg had significant increases in posttransplantation mortality with a hazard ratio of 1.58.22 However, interestingly they did not find an association between presence of HPS and waitlist survival in patients. Schenk, et al.8 followed 111 patients of compensated cirrhosis. At baseline HPS was present in 24% patients. They found a significant association between patient survival and HPS even after adjusting for Child-Pugh score. In subgroup analyses, HPS increased mortality in Child class C patients only. In their study patients with HPS had median survival 10.6 months as compared to patients without HPS who had median survival of 40.8 months. Besides HPS and Child-Pugh class, age and blood urea nitrogen were also independently associated with survival. Similar findings were observed in a study by Fallon, et al.6 in which HPS significantly increased risk of death in patients with cirrhosis. Interestingly disease severity as measured by MELD score was associated neither with HPS nor with survival in their study. In addition dyspnea, orthopnea, cyanosis, clubbing and asterixis were significantly more frequent in those with HPS compared with those without HPS.
Currently no pharmacological therapy is available for HPS. Liver transplantation is the only definitive treatment. In a prospective study on HPS patients, 5-year survival was 76% for those who underwent liver transplantation as compared to 23% for those who did not had liver transplantation.23 Even after liver transplantation, mortality is higher in patients with HPS than in patients without HPS.24 There are conflicting reports on the effect of HPS on survival in patients with cirrhosis and more studies are needed with patients from all ends of spectrum and ideally uniformly distributed in the severity of cirrhosis as per Child Pugh classification and HPS.6,8,17,20–24
Our study is the first of its kind in our region on patients with cirrhosis. Our population characteristics are quite different from what are currently reported in western countries because our patients are younger at the time of diagnosis of cirrhosis and chronic hepatitis C is the main cause of cirrhosis. One of the strengths of our study is patient selection. Our sample represented patients from all three classes of Child-Pugh score because we recruited both admitted and out-patients. That gave our study diversity with regard to disease severity. And consequently we were able to demonstrate HPS in each Child-Pugh class although HPS was significantly more common in Child class C. Limitations of our study are large number of patients being lost to follow up and relatively small follow up period. Despite these limitations, our study demonstrated that disease severity affects mortality in patients with cirrhosis and increased mortality observed in HPS patients in unadjusted Cox regression model may be due to confounding by disease severity because HPS is observed mainly in patients with advanced liver disease.
ConclusionsHPS is common in advanced liver disease and its presence correlates with MELD and Child-Pugh scores. HPS is not associated with mortality in patients with cirrhosis when adjustment is made for severity of liver disease.
Abbreviations- •
CI: confidence interval.
- •
CLD: chronic liver disease.
- •
HPS: hepatopulmonary syndrome.
- •
HR: hazard ratio.
- •
MELD: Model for End Stage Liver Disease.
- •
NO: nitric oxide.
- •
UGIB: upper gastrointestinal bleeding.
None.
AcknowledgementsNone.