Amanita phalloides is the most relevant mushroom intoxication leading to acute liver failure. The two principal groups of toxins, the amatoxins and the phallotoxins, are small oligopeptides highly resistant to chemical and physical influences. The amatoxins inhibit eukaryotic RNA polymerase II causing transcription arrest affecting mainly metabolically highly active cells like hepatocytes and renal cells. The clinically most characteristic symptom is a 6-40 h lag phase before onset of gastrointestinal symptoms and the rapid progression of acute liver failure leading to multi-organ failure and death within a week if left untreated. Extracorporeal albumin dialysis (ECAD) was reported to improve patient’s outcome or facilitate bridging to transplantation. In our tertiary center, out of nine intoxicated individuals from five non-related families six patients presented with acute liver injury; all of them were treated with ECAD using the MARS® system. Four of them were listed on admission for high urgency liver transplantation. In addition to standard medical treatment for Amanita intoxication we initiated ECAD once patients were admitted to our center. Overall 16 dialysis sessions were performed. All patients survived with full native liver recovery without the need for transplantation. ECAD was well tolerated; no severe adverse events were reported during treatment. Coagulopathy resolved within days in all patients, and acute kidney injury in all but one individual. In conclusion, ECAD is highly effective in treating intoxication with Amanita phalloides. Based on these experiences we suggest early initiation and repeated sessions depending on response to ECAD with the chance of avoiding liver transplantation.
Acute liver failure (ALF) may be caused by a wide range of conditions such as toxins, medications, ischemia, venous and bile obstruction, and genetic disorders.1 The most common mushroom intoxication leading to acute liver failure is caused by ingestion of Amanita phalloides or some other species of the genus Amanita, as well as a few other, closely related genera of basidiomycetes, which all can sometimes be mistaken for wild champignons or other edible mushrooms.2,3
The Amanita mushroom contain two principal toxin groups, the heptapeptide phallotoxins and the octapeptide amatoxins, both cyclic peptide toxins synthesized as 35 aa-long proproteins.2,3 The three most important amatoxins α-, β- and γ-amanitin have molecular masses between 0.88 and 0.92 kDa, are resistant to heat, low pH, proteases and are soluble in alcohol and water. While the phallotoxins are not taken up by the enteral mucosa resulting only in early gastrointestinal symptoms, the amatoxins are readily taken up into the enterohepatic circulation, cross cytoplasmic membranes and inactivate RNA polymerase II, leading to inhibition of transcription especially in metabolically very active cells like liver and kidney cells, which leads to cell death and in case of the liver, to rapid, progressive organ failure.3 Intoxication provokes four distinct clinical phases: a lag phase devoid of any symptoms, in contrast to many other mushroom intoxications, occurring for 6 to 40 h before onset of the gastrointestinal phase, lasting 12 to 24 h characterized by nausea, crampy abdominal pain, vomiting, diarrhea as well as consequent electrolyte imbalances, dehydration, hypoglycemia, and hypotension. After a phase of apparent convalescence (36-48 h after ingestion) with intermittent clinical improvement and characterized by deterioration of liver enzyme tests with raising transaminase levels culminating in jaundice the intoxication progresses into the phase of acute liver failure (4-9 d) characterized by dramatic raises in transaminase levels and deterioration of liver and kidney function. These conditions then lead to hyperbilirubinemia, coagulopathy, hypoglycemia, acidosis, hepatic encephalopathy, and hepatorenal syndrome. Ultimately, multi organ failure and death may occur after 1-3 weeks.4 Therefore, OLT is the treatment of choice in cases with ALF.
ECAD methods such as the Molecular Adsorbent Recirculating System (MARS®)5 or the Fractionated Plasma Separation and Adsorption System (FPSA, Prometheus®),6,7 have repeatedly shown to improve patient outcome in acute liver failure caused by intoxications. ECAD may create a time bridge until an appropriate donor organ becomes available for OLT or for avoiding the need for transplantation at all.8-11 The governing principle of these albumin dialysis methods is the selective removal of albumin-bound liver toxins in addition to removal of hydrophilic small molecules via a conventional dialysis loop.10 Rapid urinary and fecal clearance of amatoxins and the importance of sustained aggressive IV hydration therapy have been reported in amanitin intoxication.12,13,31 It is also suggested that the amatoxins themselves do not bind to albumin.14 MARS® employs an albumin-impermeable membrane with a pore size cut-off at 60 kDa against a continuously circulating loop with 20% human serum albumin passing through columns of charcoal, an anion exchange resin and a low-flux dialyzer connected to a secondary circuit.8,10 FPSA on the other hand uses a larger filter pore size (cut-off at 250 kDa) which allows the patient’s own albumin to get in contact with two filter columns’ while water-soluble substances are removed directly from blood by a separate high-flux dialysis loop.9,10 The availability of using MARS is very limited due to various reasons including deficits in evidence-based outcome data, regulatory aspects and reimbursement issues in many countries. Therefore, this system may not be available for many patients suffering from amanitin intoxication.
There are several reports on MARS® dialysis in ALF. Camus, et al. reported on a transplant-free recovery applying the MARS® system in acute liver failure.15,16 In addition’ the Helsinki group published their experience for using MARS® in amatoxin intoxication. The concept of initiating this dialysis treatment before signs of ALF developed resulted also in less need for liver transplantation.17 Additional data were published by Cisneros-Garza, et al. confirming these experiences in native liver recovery in ALF patients in a multicentric study in several Mexican hospitals.18
Here we report on our experience of employing ECAD in treatment of severe mushroom poisonings at our center from October 2010 to August 2014. Early initiation of MARS® dialysis resulted in survival without any need for OLT. The treatment was tolerated well by the patients. No clinical relevant side effects were observed.
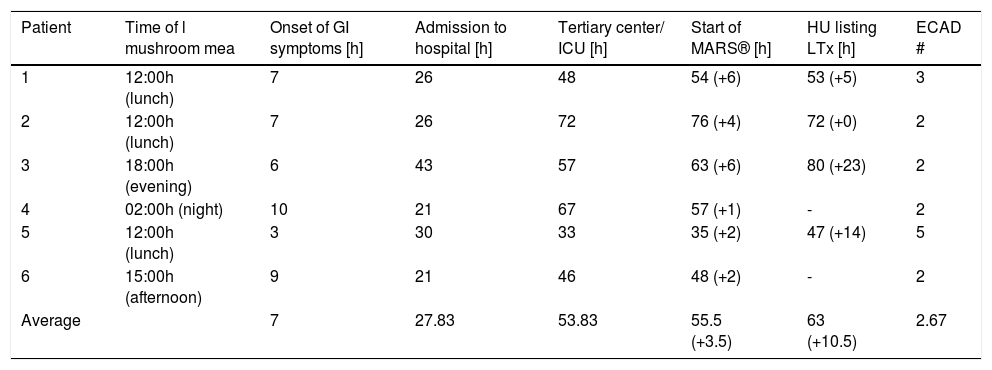
Material and MethodsPatientsBetween late summer 2010 and August 2014 nine intoxications were reported within five independent families leading to admission to a local hospital fulfilling the criteria of i) suspected amanitin intoxication and ii) predicted acute liver failure. In all cases, Amanita intoxication was confirmed by the patients, in some cases with the help of a mycologist. Out of these nine cases, three female and three male patients were transferred thereafter to our university hospital due to rapid increase of INR > 1.5. The total mean time intervals between ingestion of the mushrooms and onset of gastrointestinal (GI) symptoms were 7 h (range 3-10 h), to local hospital admission 27.83 h (range 21-43 h) and to commencement of ECAD 55.5 h (range 35-76 h). The mean admission-toMARS® time for our center was 3.5 h (range 1-6 h) and for admission-to-HU listing the interval was 10.5 h (0-23 h) (Table 1). Mean age was 56.8 years (range 34-78 years). Mean number of ECAD treatments were 2.67 (range 2-5), mean duration was 6.63 h (range 1.08-13.84 h) and mean length of total university hospital stay was 13.38 d (range 4-36 d). Tables 2A-2F illustrate fluid balances, blood gas analysis and laboratory parameters of the presented patients. Our standard medical treatment for amanitin intoxications for all six individuals during their ICU stay and at the day of discharge is outlined in table 3. Four of the six patients were initially listed for high urgent liver transplantation, but all could be de-listed afterwards without fatalities (Figure 1). Due to comorbidity, age or rapid clinical improvement two patients were not listed for liver transplantation despite initial presentation with acute liver injury.
Total time intervals between ingestion, hospital and ICU admissions, HU listing and initiation of ECAD.
| Patient | Time of l mushroom mea | Onset of GI symptoms [h] | Admission to hospital [h] | Tertiary center/ ICU [h] | Start of MARS® [h] | HU listing LTx [h] | ECAD # |
|---|---|---|---|---|---|---|---|
| 1 | 12:00h (lunch) | 7 | 26 | 48 | 54 (+6) | 53 (+5) | 3 |
| 2 | 12:00h (lunch) | 7 | 26 | 72 | 76 (+4) | 72 (+0) | 2 |
| 3 | 18:00h (evening) | 6 | 43 | 57 | 63 (+6) | 80 (+23) | 2 |
| 4 | 02:00h (night) | 10 | 21 | 67 | 57 (+1) | - | 2 |
| 5 | 12:00h (lunch) | 3 | 30 | 33 | 35 (+2) | 47 (+14) | 5 |
| 6 | 15:00h (afternoon) | 9 | 21 | 46 | 48 (+2) | - | 2 |
| Average | 7 | 27.83 | 53.83 | 55.5 (+3.5) | 63 (+10.5) | 2.67 |
In parenthesis: relative time intervals after admission to our center.
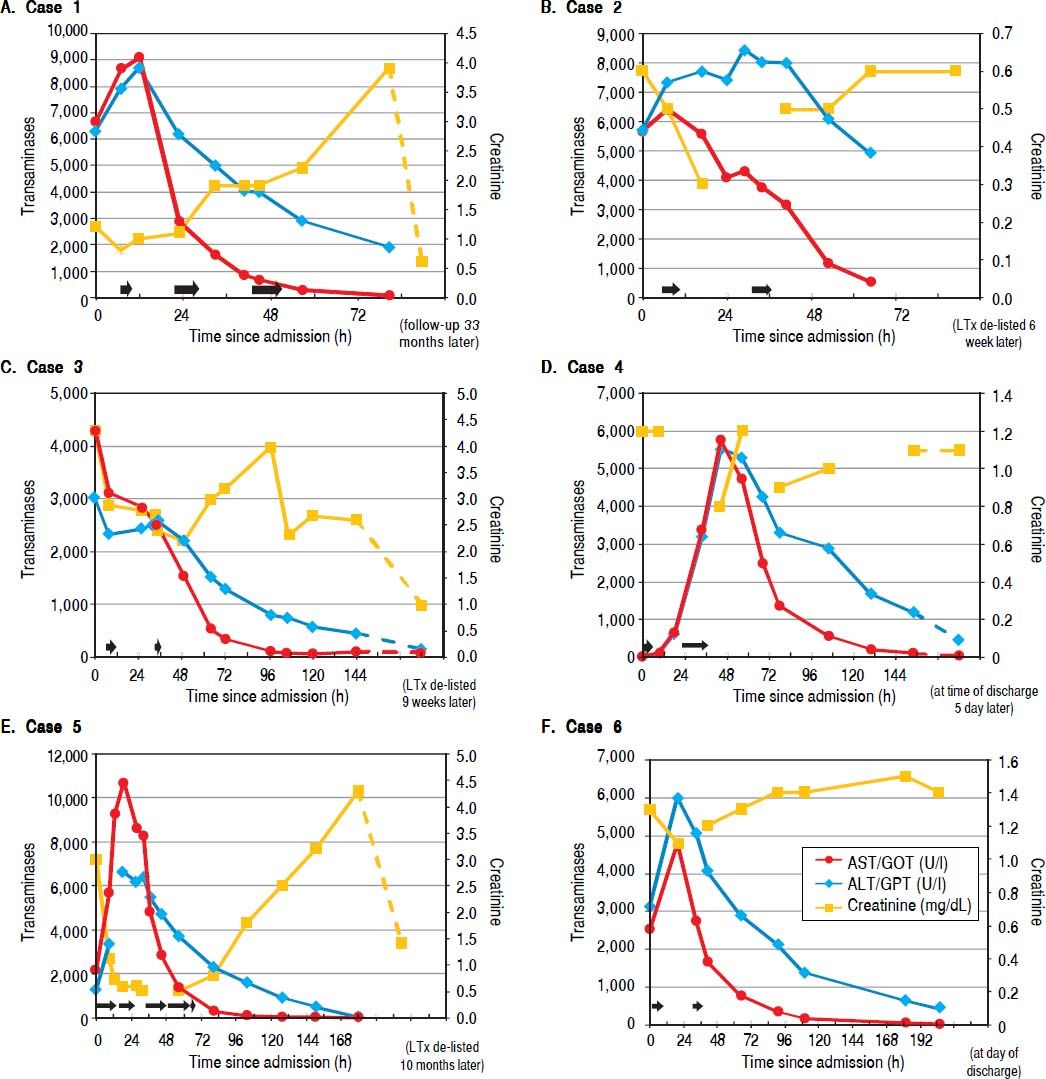
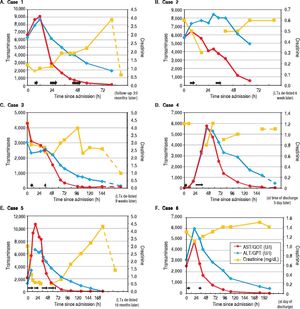
Liver parameters over time after admission to our hospital are illustrated. On each graph the left y-axis depicts transaminase enzyme activities in units per liter (ALT/GPT, alanine aminotransferase; AST/GOT, aspartate aminotransferase). The righty-axs displays serum creatinine in milligrams per deciliter. The horizontal black arrows denote each ECAD treatment and its duration.
For MARS® treatment we used a Fresenius 5008 or a Genius dialysis machine (Fresenius Medical Care Deutschland GmbH, 61346 Bad Homburg v. d. H.) and a MARS® Monitor (Gambro Lundia AB, Sweden). A conventional hemodialysis catheter access either through the jugulary or the subclavian vein was established. To prevent clotting in the albumin-impregnated, highly permeable dialyzer system we used a continuous infusion of unfractionated low molecular weight heparin (Ratiopharm GmbH, Ulm, Germany). The closed loop contained 500 ml of 20% commercial human serum albumin. The flow in the blood loop, the albumin dialysate circuit and the low-flow albumin-regeneration dialyzer were kept at 250 mL/min.
Safety monitoringThe most frequently reported serious adverse events due to ECAD in medical literature are bleeding due to anticoagulation and/or thrombocytopenia.19 Platelets may drop during and after ECAD in individual cases.19
Blood pressure and heart rate were continuously monitored during the ECAD sessions, while laboratory measurements were taken at least daily. Routine creatinine measurements were taken photometrically, as there are currently no official recommendations to use the enzymatical method in MELD calculations.20
ResultsCase 1A 56-year-old woman was admitted to a local hospital after having ingested a mushroom meal at lunch together with her family the day before. Her medical history included arterial hypertension and one historic episode of pyelonephritis. In the evening, about 6 h after the meal, she started to suffer from diarrhea, nausea, vomiting and leg cramps. Her husband and two of her children developed similar, but milder symptoms. Together, the family was admitted to a hospital. All except her daughter (see case 2) developed transaminases below 1000 U/l and remained clinically stable. A mycologist examined remaining samples from the rest of the mushroom meal and discovered several mildly gastrointestinal-toxic fungi including Amanita species, but could not detect A. phalloides anymore. After initiation of treatment for Amanita intoxication with silibinin, charcoal and fluid resuscitation the patient developed fever, raising transaminase values and falling liver synthesis parameters. The patient was then transferred to the ICU of our tertiary care center for highly urgent (HU) liver transplantation listing. Sonography revealed hepatomegaly with parenchymal damage without cholestasis, while CT scanning showed no necrotic foci within the liver at that early stage.
In addition to standard medical treatment (Table 3) the patient received three ECAD therapies on three consecutive days, which resulted in reversal of transaminases and normalization of parameters for liver synthesis (Figure 1A, Table 2A). The clinical condition of the patient improved markedly, she was taken off the high urgency OLT list. Acute kidney injury occurred accompanied by anuria, which resolved within days (Table 2A). She was hospitalized for 4.3 days.
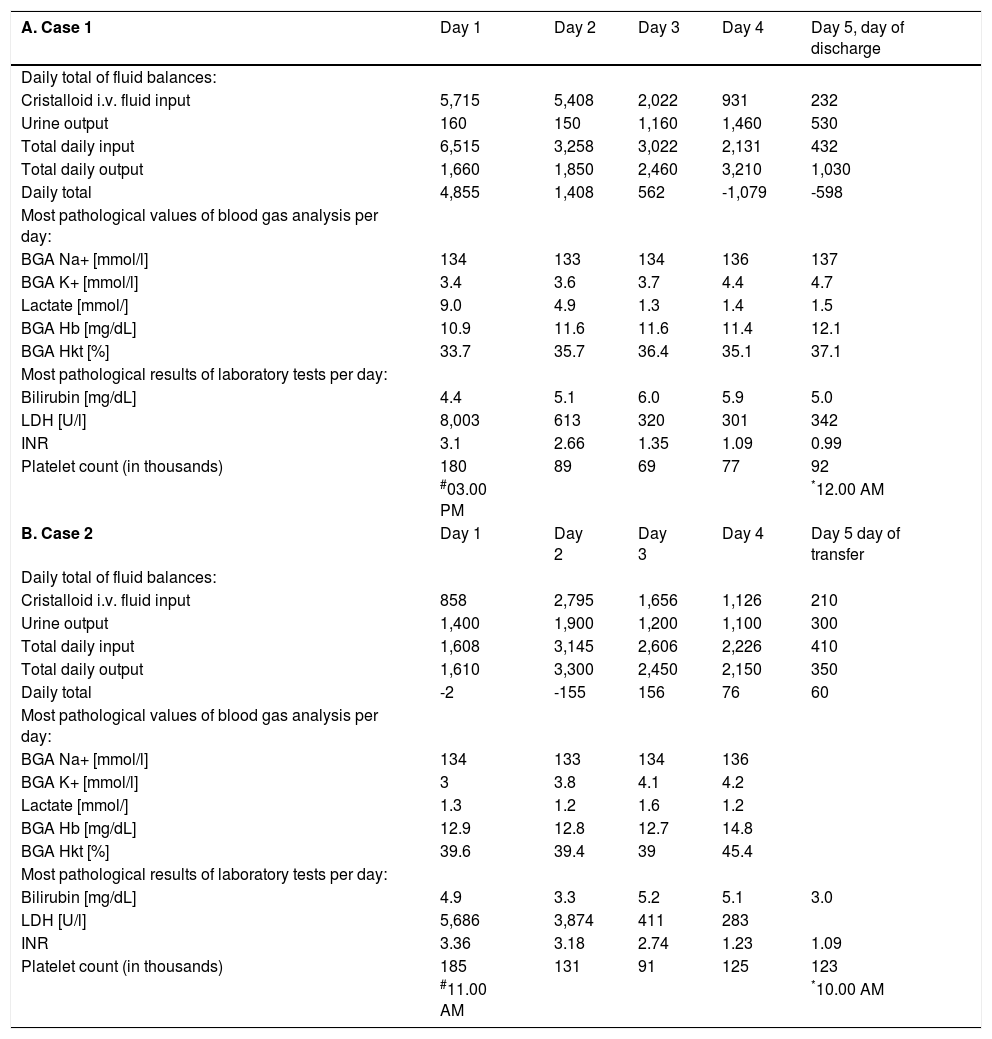
A-F. Fluid balances (*per 24 h, at 6.00 a.m.; in millilitres), blood gas analysis and laboratory parameters (most pathological values for each respective day) for all six patients at admission and during ICU treatment for the first seven days, or until transfer to the normal ward.
| A. Case 1 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5, day of discharge |
|---|---|---|---|---|---|
| Daily total of fluid balances: | |||||
| Cristalloid i.v. fluid input | 5,715 | 5,408 | 2,022 | 931 | 232 |
| Urine output | 160 | 150 | 1,160 | 1,460 | 530 |
| Total daily input | 6,515 | 3,258 | 3,022 | 2,131 | 432 |
| Total daily output | 1,660 | 1,850 | 2,460 | 3,210 | 1,030 |
| Daily total | 4,855 | 1,408 | 562 | -1,079 | -598 |
| Most pathological values of blood gas analysis per day: | |||||
| BGA Na+ [mmol/l] | 134 | 133 | 134 | 136 | 137 |
| BGA K+ [mmol/l] | 3.4 | 3.6 | 3.7 | 4.4 | 4.7 |
| Lactate [mmol/] | 9.0 | 4.9 | 1.3 | 1.4 | 1.5 |
| BGA Hb [mg/dL] | 10.9 | 11.6 | 11.6 | 11.4 | 12.1 |
| BGA Hkt [%] | 33.7 | 35.7 | 36.4 | 35.1 | 37.1 |
| Most pathological results of laboratory tests per day: | |||||
| Bilirubin [mg/dL] | 4.4 | 5.1 | 6.0 | 5.9 | 5.0 |
| LDH [U/l] | 8,003 | 613 | 320 | 301 | 342 |
| INR | 3.1 | 2.66 | 1.35 | 1.09 | 0.99 |
| Platelet count (in thousands) | 180 | 89 | 69 | 77 | 92 |
| #03.00 PM | *12.00 AM | ||||
| B. Case 2 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 day of transfer |
| Daily total of fluid balances: | |||||
| Cristalloid i.v. fluid input | 858 | 2,795 | 1,656 | 1,126 | 210 |
| Urine output | 1,400 | 1,900 | 1,200 | 1,100 | 300 |
| Total daily input | 1,608 | 3,145 | 2,606 | 2,226 | 410 |
| Total daily output | 1,610 | 3,300 | 2,450 | 2,150 | 350 |
| Daily total | -2 | -155 | 156 | 76 | 60 |
| Most pathological values of blood gas analysis per day: | |||||
| BGA Na+ [mmol/l] | 134 | 133 | 134 | 136 | |
| BGA K+ [mmol/l] | 3 | 3.8 | 4.1 | 4.2 | |
| Lactate [mmol/] | 1.3 | 1.2 | 1.6 | 1.2 | |
| BGA Hb [mg/dL] | 12.9 | 12.8 | 12.7 | 14.8 | |
| BGA Hkt [%] | 39.6 | 39.4 | 39 | 45.4 | |
| Most pathological results of laboratory tests per day: | |||||
| Bilirubin [mg/dL] | 4.9 | 3.3 | 5.2 | 5.1 | 3.0 |
| LDH [U/l] | 5,686 | 3,874 | 411 | 283 | |
| INR | 3.36 | 3.18 | 2.74 | 1.23 | 1.09 |
| Platelet count (in thousands) | 185 | 131 | 91 | 125 | 123 |
| #11.00 AM | *10.00 AM |
| C. Case 3 | Day 1 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|
| Daily total of fluid balances: | ||||||
| Cristalloid i.v. fluid input | 4,120 | 3,000 | 2,901 | 2,321 | 2,172 | 2,368 |
| Urine output | 1,130 | 840 | 1,850 | 1,120 | 2,000 | 2,900 |
| Total daily input | 8,370 | 5,200 | 5,551 | 3,371 | 4,372 | 3,768 |
| Total daily output | 3,080 | 940 | 2,900 | 2,540 | 6,100 | 3,670 |
| Daily total | 2,060 | 4,260 | 2,650 | 831 | -1,728 | 89 |
| Most pathological values of blood gas analysis per day: | ||||||
| BGA Na+ [mmol/l] | 133 | 132 | 134 | 137 | 138 | |
| BGA K+ [mmol/l] | 3.6 | 2.7 | 4.0 | 4.2 | 3.8 | |
| Lactate [mmol/] | 3.8 | 3.2 | 3.0 | 2.9 | 2.5 | |
| BGA Hb [mg/dL] | 10.6 | 10.1 | 10.7 | 11.3 | 10.3 | |
| BGA Hkt [%] | 32.8 | 33.5 | 33 | 34.7 | 32.4 | |
| Most pathological results of laboratory tests per day: | ||||||
| Bilirubin [mg/dL] | 3.9 | 5.3 | 12.3 | 14.7 | 23 | 22.1 |
| LDH [U/l] | 2,491 | 2,003 | 406 | 348 | 353 | |
| INR | 4.38 | 6.14 | 2.3 | 1.53 | 1.4 | 1.3 |
| Platelet count (in thousands) | 107 | 20 | 6 | 11 | 19 | 17 |
| #01.00 AM | *24.00 AM |
| D. Case 4 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| Daily total of fluid balances: | |||||
| Cristalloid i.v. fluid input | 824 | 1,453 | 542 | ||
| Urine output | 350 | 2,150 | 350 | ||
| Total daily input | 1,474 | 3,853 | 1,392 | ||
| Total daily output | 650 | 3,300 | 650 | ||
| Daily total | 824 | 553 | 742 | ||
| Most pathological values of blood gas analysis per day: | |||||
| BGA Na+ [mmol/l] | 136 | 134 | 135 | ||
| BGA K+ [mmol/l] | 3.6 | 3.4 | 3.4 | ||
| Lactate [mmol/] | 1.4 | 1.8 | 1.2 | ||
| BGA Hb [mg/dL] | 11.1 | 10.8 | 11.2 | ||
| BGA Hkt [%] | 34.2 | 33.4 | 34.5 | ||
| Most pathological results of laboratory tests per day: | |||||
| Bilirubin [mg/dL] | 1.1 | 2.1 | 2.8 | 2.7 | 2.6 |
| LDH [U/l] | 259 | 624 | 4,790 | 785 | 345 |
| INR | 1.05 | 1.35 | 2.14 | 2.15 | 1.54 |
| Platelet count (in thousands) | 253 | 229 | 97 | 82 | 54 |
| 07.00 AM | *10.00 PM | ||||
| E. Case 5 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| Daily total of fluid balances: | |||||
| Cristalloid i.v. fluid input | 3,704 | 2,769 | 2,716 | 2,806 | 495 |
| Urine output | 500 | 570 | 3,350 | 3,900 | 500 |
| Total daily input | 4,101 | 4,519 | 4,016 | 5,356 | 695 |
| Total daily output | 1,900 | 2,820 | 5,100 | 5,800 | 500 |
| Daily total | |||||
| Most pathological values of blood gas analysis per day: | |||||
| BGA Na+ [mmol/l] | 139 | 134 | 133 | 133 | 133 |
| BGA K+ [mmol/l] | 3.0 | 2.5 | 3.4 | 3.5 | 3.6 |
| Lactate [mmol/] | 2.9 | 2.4 | 1.7 | 0.8 | 0.8 |
| BGA Hb [mg/dL] | 16.4 | 12.4 | 9.8 | 9.0 | 9.8 |
| BGA Hkt [%] | 50.1 | 38.2 | 30.3 | 28 | 30.3 |
| Most pathological results of laboratory tests per day: | |||||
| Bilirubin [mg/dL] | 0.7 | 1.4 | 2.8 | 3.3 | 3 |
| LDH [U/l] | 1,924 | 8,398 | 543 | 273 | 258 |
| INR | 1.01 | 1.64 | 1.35 | 0.94 | 0.91 |
| Platelet count (in thousands) | 327 | 277 | 70 | 65 | 75 |
| #08.00 PM | *07.00 PM | ||||
| F. Case 6 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| Daily total of fluid balances: | |||||
| Cristalloid i.v. fluid input | 1963 | 617 | 282 | ||
| Urine output | 300 | 950 | 1,800 | ||
| Total daily input | 2,683 | 3,067 | 2,782 | ||
| Total daily output | 500 | 1,300 | 2,150 | ||
| Daily total | 2,183 | 1,767 | 632 | ||
| Most pathological values of blood gas analysis per day: | |||||
| BGA Na+ [mmol/l] | 134 | 133 | 132 | 133 | |
| BGA K+ [mmol/l] | 3.8 | 3.6 | 3.6 | 3.8 | |
| Lactate [mmol/] | 1.4 | 1.6 | 1.2 | 0.8 | |
| BGA Hb [mg/dL] | 11.4 | 10.1 | 9.9 | 10.1 | |
| BGA Hkt [%] | 35 | 31.4 | 30.5 | 30.5 | |
| Most pathological results of laboratory tests per day: | |||||
| Bilirubin [mg/dL] | 2.1 | 1.7 | 2.0 | 1.7 | 1.3 |
| LDH [U/l] | 2,196 | 3,513 | |||
| INR | 1.62 | 1.87 | 1.43 | 1.27 | 1.08 |
| Platelet count (in thousands) | 240 | 197 | 162 | 153 | |
| #13.00 PM | *05.00 AM |
Standard medical treatment: intoxication-specific and general medication orders for all six cases during ICU stay and at day of discharge.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Anti-intox. (all i.V., except noted otherwise) | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine thiamine | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine | charcoal (p.o.), silibinine (Legalon®), vitamin K, acetylcysteine |
| Gastroenterological (all p.o., except noted otherwise) | ranitidine, metoclopramide, magaldrate (Riopan® Gel) | lactulose, metoclopramide, dimeticone (Sab simplex®), sodium sulfate decahydrate (Glauber’s salt), butylscopolamine | pantoprazole, metoclopramide | metoclopramide, butylscopolamine, alizapride, lactulose, ranitidine | dimenhydrinate, lactulose, pantoprazole | |
| Cardiological (all i.v., except noted otherwise) | urapidile, noradrenaline | glyceryl trinitrate, urapidile, lercandipine (p.o.), Clonidine | Enalaprile, urapidile, glyceryl trinitrate | urapidile, amlodipine (p.o.), ramiprile (p.o.), glyceryl trinitrate | glyceryl trinitrate, ramiprile (p.o.), urapidile, Clonidine, lercandipine (p.o.) | |
| Nephrological (all i.v., except noted otherwise) | potassium chloride, sodium chloride, Sterofundin ISO® | potassium chloride, sodium chloride, Sterofundin ISO®, Potassium chloride | potassium chloride, sodium chloride, Sterofundin ISO®, Potassium chloride | potassium chloride, sodium chloride, Sterofundin ISO® | Sterofundin ISO®, potassium chloride, sodium chloride, torasemide | Sterofundin ISO®, potassium chloride, sodium chloride |
| Pain (all i.V., except noted otherwise) | metamizole | metamizole | piritramide, metamizole, morphine | metamizole | metamizole | |
| Metabolic (all i.V., except noted otherwise) | glucose/sodium chloride 40% | glucose/sodium chloride 40% | glucose/sodium chloride 40%, Olimel emulsion | glucose/sodium chloride 40% | insulin, glucose/sodium chloride 40% | insulin, glucose/sodium chloride 40% |
| Antibiotic (all i.v., except noted otherwise) | ceftriaxone | piperacilline-tazobactam, ceftriaxone | meropenem | |||
| Other (all i.v., except noted otherwise) | Aminoplasmal Hepa®, thiamine, nicotine patch | Aminoplasmal Hepa® | freshly frozen plasma, human albumin | |||
| Day of discharge (all p.o.) | heparin, ranitidine, metamizole | metoprolole, amlodipine | enalapril, amlodipine, pantoprazole | amlodipine, ramiprile, ranitidine, lactulose | ramiprile, lercandipine, pantoprazole |
The 34-year-old daughter of patient #1 developed a similar rise in transaminases, albeit several hours later than her mother. This patient had no relevant medical history. She had ingested the mushroom meal together with her mother (see case 1 for details), and was transferred from the local hospital to our tertiary center one day later, suffering from the same clinical symptoms as her mother with laboratory findings consistent with hyperacute liver injury. Onset of clinical symptoms in her case was about 11 h after the meal. She received the same supportive medical treatment (Table 3) as her mother and was also listed for HU OLT, but after two sessions of ECAD her lab values had already improved markedly and she could be taken off the waiting list (Figure 1B, Table 2B). Without any signs of acute kidney injury and in markedly improved clinical condition she was transferred back to her local hospital (Figure 1B). Hospitalization was overall 3.95 days.
Case 3A 47-year-old man presented with abdominal pain, nausea and diarrhea 6 h after ingesting a meal of self-collected mushrooms for dinner. He had no history of liver disease. On the next day, he was admitted to a local hospital, was then transferred to a secondary care hospital because of rising transaminase values to initiate treatment for amanitin intoxication with silibinin and N-acetylcysteine (Table 3). Due to further deteriorating lab tests and additional onset of acute kidney injury with edema of the lower extremities the patient was quickly transferred to our university hospital to initiate ECAD therapy and possible HU listing for OLT. Ultrasound revealed signs of hepatic parenchymal swelling without signs of obstructions. After two MARS® sessions ECAD was suspended because of suspected heparin induced thrombocythaemia type II (HIT II) and HU OLT listing was carried out. At time of a donor offer, the patient had already improved liver laboratory values and therefore no OLT was required anymore. Subsequently, his renal function also returned to normal values (Figure 1C, Table 2C). The patient was discharged after 15.35 days from our hospital in good general condition.
Case 4A 67-year-old man with a medical history of arterial hypertension, hepatitis B, hyperthyroidism and cachexia was admitted to our emergency department and then transferred directly to our hospital’s ICU because of an Amanita mushroom poisoning with subsequent acute liver injury. Approximately 10 h after completely ingesting a meal of self-collected mushrooms (his description of one of them resembled a white death cap), he developed nausea, emesis, watery diarrhea and gastric pain. After admission we began intensive care treatment with standard medical therapy for Amanita intoxication with charcoal, silibinin, N-acetylcysteine (Table 3) and carried out two supporting ECAD sessions. He required continuous antihypertensive medication with urapidil and sublingual vasodilatators. His transaminase values decreased rapidly; therefore HU OLT listing was not carried out (Figure 1D, Table 2D). Overall the patient was 10.63 days hospitalized.
Case 5A 58-year-old woman with a medical history of several episodes of cholelithiasis, breast cancer and arterial hypertension was transferred from a local hospital to our Intensive Care Unit due to acute liver and kidney injury 33 hours after Amanita intoxication. She had developed nausea, emesis and diarrhea after eating the larger part of a meal of mushrooms which all had been collected by relatives, while her husband took the smaller portion. He was only briefly hospitalized with the same but milder symptoms. She presented with extensively elevated liver and kidney retention parameters. After admission to our ICU a therapy with silibinin, charcoal and lactulose was initiated, together with antihypertensive intravenous urapidil treatment (Table 3). She was promptly listed for HU OLT. ECAD was administered five times in four days until liver and coagulation parameters had improved. After albumin dialysis was discontinued, hemodialysis was reintroduced since renal parameters increased again. Dialysis was performed three times until discharge, 15 days after intoxication.
Two weeks later, serum creatinine levels had fallen to about 1.3 mg/dl under constant ambulatory surveillance (Figure 1E, Table 2E). About eight months after discharge her creatinine levels had returned to almost normal values. Hospital length of stay was 36 days.
Case 6A 78-year-old man with a medical history of arterial hypertension and tuberculosis many years ago was admitted to a local hospital with nausea as well as elevated liver parameters after ingestion of self-collected mushrooms on the afternoon of the day before. During the night he had developed severe gastroenteritic symptoms with severe emesis and diarrhea. After initiation of standard medical treatment (penicillin G, activated charcoal, silibinin) in the local hospital the patient developed a dramatic increase in transaminases and decrease in coagulation parameters. He was rapidly transferred to our hospital’s ICU and received two ECAD treatments in addition to our standard of care (Table 3). His liver parameters started to decrease, the prothrombin time began to rise slowly and the renal parameters remained stable (Figure 1F, Table 2F). One week later, the patient was discharged from our internal medicine ward in good condition with only creatinine levels still elevated above normal values. His serum creatinine levels already had been at 1.3 mg/dL eight months before the intoxication and remained elevated at 1.4 mg/dL six months later. Total length of stay in our hospital was 10 days.
OutcomeAmong nine intoxicated individuals, six were admitted to our center for OLT due to acute liver injury. All of these patients fulfilled the criteria for amanitin intoxication and predicted acute liver failure. Four of these six patients developed acute kidney injury, one patient (case 6) already had elevated serum creatinine values before the intoxication and developed only a mild decrease in renal function, and another never showed any increase in kidney retention parameters. Oliguria was detectable before any changes in serum creatinine were noticed. Volume expansion was used in all patients to treat or prevent pre-renal acute kidney failure (Tables 2A-2F, Table 3). Both liver and kidney injury parameters recovered in all patients to their initial values, except for case 5, who retained slightly elevated serum creatinine values afterwards. Transaminases always recovered within the very first days, whereas bilirubin and renal function recovered usually within days to a few weeks after intoxication. Since all patients had no history of both liver and renal disease, transaminases seemed to be the best parameter to predict the time course of the disease. Nevertheless, MARS® dialysis may be even continued until bilirubin is decreasing as long as the treatment is tolerated. We use a drop of platelets below 50.000/u.L as a trigger to stop albumin dialysis. Any decrease in bilirubin may enhance regeneration of the liver after ECAD has been started. Therefore, MARS® may be continued even longer to increase patient benefit. MARS® therapy was well tolerated in all six patients; however, drop of platelets, bleeding events or infections as well as the logistics and general availability of this form of dialysis may limit their usage. No patient required OLT although four out of six patients were listed HU. All organ offers were rejected due to fast recovery of medical status and patients were delisted subsequently.
Safety and adverse events during treatmentTo assess the patient’s condition we at least daily monitored serum electrolytes, blood urea nitrogen, serum creatinine, total bilirubin, transaminases (AST/GOT, ALT/GPT), lactate dehydrogenase, prothrombin time, International Normalized Ratio (INR), partial prothrombin time (PTT), albumin, and blood cell counts. Only platelet counts significantly deteriorated during the ECAD treatment in each patient. All other determined laboratory parameters benefited from ECAD. The most important parameters are shown in figures 1A-1F and tables 2A-2F.
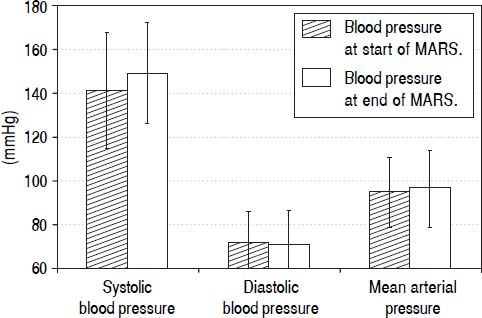
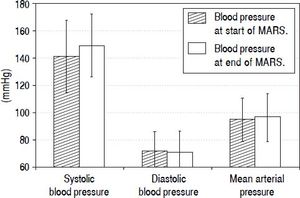
During each MARS® treatment we continuously monitored arterial blood pressure (Figure 2). In this analysis we included all patients with at least two or more blood pressure measurements taken and without any antihypertensive medication (12 out of 16 sessions fulfilled these criteria). The first measured mean systolic blood pressure value was 141.42 ± 26.32, diastolic pressure was 71.67 ± 14.46 (MAP 94.92 ± 15.97) and the last measured mean systolic value was 149.08 ± 22.98 with a diastolic value of 71.08 ± 15.28 (MAP 97.08 ± 16.84). Therefore, blood pressure during ECAD remained stable in contrast to other intermittent dialysis methods reported.
Mean arterial blood pressure values during 12 out of a total of 16 ECAD treatments in six patients with Amanita phalloides intoxication taken during routine monitoring of vital parameters at the beginning and at the end of MARS® sessions. Data from sessions with less than two blood pressure measurements or under continuous antihypertensive medication during the sessions were omitted from analysis (4 out of 16 treatments).
In this work we present our tertiary care center’s experience in treatment of Amanita intoxication with extracorporeal albumin dialysis from October 2010 till August 2014. Out of nine individuals from five families exposed to varying amounts of poisonous mushrooms, six patients were predicted to develop symptoms of acute liver failure and had to be transferred from their respective local hospitals to our liver transplant center. In all six cases liver support therapy was started almost immediately on admission (Table 1). The liver parameters of all patients improved during the next 2-4 days of treatment. Four patients were eligible for HU listing, whereas two patients were not listed due to age, comorbidity or clinical improvement. In all patients, coagulopathy resolved itself during two to three days of ECAD treatment. Since patients seemed to be stable under MARS® therapy, organ offers from ET were declined and later on patients were delisted due to full recovery. HU listing in all four cases was confirmed by an audit committee of Eurotransplant (Leiden, The Netherlands).
Kidney retention parameters stayed stable in two patients or returned to the normal range in one case within weeks. First renal symptom was oliguria (Tables 2A-2F). Two patients developed acute renal failure during their ICU stay requiring continuous veno-venous hemodialysis (CVVH), necessitating temporary ambulatory renal replacement therapy until kidney function recovered. One patient already had a slightly impaired renal function eight months before the intoxication as well as six months afterwards. Transaminases returned to normal values within a few days in all patients, as did total bilirubin in three individuals before their discharge. In the other three patients the bilirubin values had almost reached normal values at discharge or at the time of a further blood test during a subsequent ambulatory counseling in our outpatient clinic (Figures 1A-1F, Tables 2A-2F).
In our experience, MARS® was generally tolerated very well by all patients without major adverse events. Transient thrombocytopenia was also present in our patients, but recovered quickly as was described previously for MARS®19,21 and Prometheus®.22 In addition, severe outcomes of amanitin intoxications like death, often described in earlier studies,23 as well as emergency liver transplantation associated with its higher intrinsic risk of peri- and post-operative mortality, could be avoided in our cohort.24
ECAD treatment for mushroom-induced acute liver failure has already been described by several groups. In 2003, Faybik, et al. published their experience with MARS® in six patients with a mean time interval between exposure and first ECAD session of 76 h, with 1-3 sessions per patient lasting between 10-24 h. No ECAD-related adverse events were reported.25 Two of their patients regenerated spontaneously, two were bridged successfully to liver transplantation (one adult died in septic shock a few days after transplantation, while one pediatric patient survived without complications). In one patient with graft dysfunction after emergency OLT of a marginal organ ECAD helped to avoid re-transplantation. One patient developed a fatal cerebral herniation shortly before transplantation was to be carried out. The authors concluded that ECAD seemed to be a successful treatment perspective for support of liver regeneration either to avoid transplantation, for bridging until Tx becomes possible, or for treatment of graft dysfunction.25
Covic, et al. published in 2003 an early series of six pediatric patients aged 7-16 years from Romania accidently intoxicated with mushrooms.26 They performed two MARS® sessions per patient lasting 6 h each without any complications. Four out of six patients survived with complete recovery of liver function. The authors concluded that ECAD was a safe and effective depurative therapy in children in acute liver failure and that survival was predicted in their study only by the impact of the initial MARS® sessions and not by baseline laboratory parameters.26
Kantola, et al. in 2009 emphasized the urgency of commencing ECAD treatment in mushroom intoxications while the patients are still in a relatively good clinical condition.21 They recorded a mean time interval of 18 h from ingestion to start of medical treatment and of 48 h until start of MARS® therapy in their series of ten adult patients from Finland. They performed a median of three ECAD sessions per patient lasting for 15.4 h on average, without any MARS®-related adverse events. Five out of six patients with laboratory signs of acute liver failure experienced native liver recovery, only one was bridged to successful OLT. The other four patients never developed any degree of liver failure prompting the authors to speculate if in retrospect ECAD was indicated at all. They concluded that early aggressive MARS® therapy in combination with optimum supportive measures seemed to be beneficial in cases of Amanita intoxication. Additionally, they also acknowledged the fact that the optimal intensity, duration and initiation criteria for ECAD in these cases have still not been developed.21
Sorodoc, et al. published in 2010 a retrospective case series of six Romanian patients of whom the first three had received ECAD plus optimal intensive care and the last three had received optimal intensive care without MARS®.27 The former group received three six-hour sessions per patient, with the first as late as 89-121 h after ingestion of the mushroom meal, without any significant adverse reactions. In the non-ECAD group, all patients died within 10 days post-intoxicationem and in the ECAD group, only one out of three individuals survived, attributed by the authors to the almost complete lack of options for emergency liver transplantations caused by severe donor organ shortage in their country. They concluded that the time point of initiation of ECAD therapy, its initial impact as well as the amatoxin inoculum size predicted the outcome of the patients in their small study.27
Recently, Cisneros-Garza, et al. reported on 38 Mexican patients with ALF (of whom 54.3% received MARS®), 15 patients with AoCLF (24.2% with MARS®) and 17 cholestatic patients with intractable pruritus (21.5% MARS®) showing significant reductions in most important liver parameters (serum bilirubin, AST, ALT, g-GT, AP) and improved grade of encephalopathy. Of the 47.3% survivors in the first group, 36.8% did not need transplantation and the other 10.5% were successfully bridged until a suitable donor organ became available. Cisneros-Garza, et al. therefore concluded that MARS® is a safe and effective procedure especially for patients with acute liver failure with the potential to contribute to native liver recovery.18
Concerning other modalities of albumin dialysis, Bergis, et al. were the first group to report on amanitin intoxications treated with the Fractionated Plasma Separation and Adsorption (FPSA, Prometheus®) system.22 They compared a group of nine patients with 1-2 FPSA cycles lasting six hours each to eleven matched patients which received standard medical therapy only. Mean time between mushroom ingestion and onset of diarrhea was 16.2 ± 4.63 h and from ingestion to hospital admission 35.7 ± 6.37 h. All patients in the FPSA group tolerated the procedures well and recovered fully without need for liver transplantation, while in the control group one patient died from shock and cardiac arrest, one patient developed renal insufficiency and one retained elevated kidney retention parameters after discharge. The authors concluded that liver support therapy by FPSA was a safe method with the potential of reducing the need for emergency liver transplantations. They also acknowledged the current lack of large controlled studies addressing the questions of the optimal detoxification system, the optimum number of treatment cycles and the identification criteria of patients that would benefit the most from liver support therapy.22
In our case series, the mean time of onset of gastrointestinal symptoms was 7 h, admission to local hospitals occurred about 28 h after ingestion. After initial treatment for on average 26 h at the local hospital, the patients were transferred to our center where ECAD was commenced with a delay of only 3 h 30 min after admission. HU listing was established on average in less than 11 h (Table 1). These time intervals of about one day to hospital admission and another one to two days until start of MARS® are comparable to the intervals reported by previous ECAD studies.21,22,25
The aforementioned reports on ECAD therapies in amatoxin-induced acute liver failure including our herein reported patients may have had a similar outcome without interventional ECAD treatments. However, based on the experience in acute or hyperacute liver failure the survival is surprisingly very impressive despite the low numbers of patients studied.
Concerning endogenous mediators of liver toxicity, recent in vitro studies utilizing an albumin dialysis model system indicated removal of water-soluble substances’ proinflammatory mediators like IL-6 and TNFα using the MARS Flux® membrane. An even better removal was achieved using membranes with larger pores.28,29 However’ the authors point out that it is still unclear whether increased or decreased levels of cytokines are important for the survival of patients.28 Concerning in vivo data from a clinical setting, Donati, et al. reported in 2013 on their experience with 64 patients and 269 MARS sessions showing that small molecules like bilirubin, ammonia and bile acids were significantly reduced and hepatocyte growth factor (HGF) concentrations improved, while cytokines were not affected by MARS.30
Our clinical experience with our strategy of ‘the earlier initiation of albumin dialysis, the better the outcome in ALF’ needs further evaluation and a better understanding of the initial pathophysiological mechanisms resulting in liver failure. Early markers for both liver and renal failure to better define timing and modes of ECAD in this setting are missing. Experience in management of using ECAD may also influence outcome. A prospective randomized study is desired. However, with our experience at our center it would be of some ethical concern to have a controlled study including a control arm offering no ECAD therapy in amatoxin induced liver failure. In addition, we provide evidence that, in contrast to the previously reported experience in other studies that dialysis tends to lower blood pressure, the MARS® system resulted in stable hemodynamics. In patients with low blood pressure, this is of interest especially in the setting of liver diseases, since blood pressure impacts renal perfusion and therefore renal excretion as well as volume balance. We observed that renal function recovered fully in all our patients to their pre-intoxication levels. While there seems to be general consensus on the early initiation of ECAD treatment, fewer consensuses exists on the duration of and the time intervals between the respective sessions.15,19,21,22,25,31
ConclusionThis study highlights the efficacy of the MARS® system in treating intoxications with Amanita phalloides. Based on these experiences we suggest early initiation, e.g. once transaminases reach 1,000 U/l or parameters such as INR increase. Repeated sessions depending on response of ECAD are required offering the chance of avoiding OLT. Platelets are critical for deciding timing and duration of MARS®. Further studies are required to determine dosage, intervals and the optimum characteristics for ECAD treatment of patients suffering from acute liver failure due to mushroom intoxication. These experiences are also relevant for other forms of liver failure, reconsidering ECAD as an optional tool in the multimodal treatment pattern in liver failure or intoxications caused by substances with or without affinity to albumin.
Abbreviations- •
ALF: acute liver failure.
- •
AoCLF: acute-on-chronic liver failure.
- •
ECAD: extracorporeal albumin dialysis.
- •
HU: high urgency.
- •
ICU: Intensive Care Unit.
- •
MARS®: molecular adsorbent recirculating system.
- •
OLT: orthotopic liver transplantation.
- •
Tx: transplantation.
The authors declare that there are no conflicts of interest.
Informed ConsentAll procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
ContributionsMHP collected the data, prepared the figures, reviewed the literature and prepared the manuscript. TS contributed in data collection and helped in preparing the manuscript. PB gave valuable advice on the clinical management of acute liver failure, and reviewed the manuscript together with GG and HP, who additionally offered invaluable insights into the clinical as well as technical aspects of dialysis methods. HS was responsible for all cases presented, supervised the project and helped to prepare the final draft of the manuscript.