Epidemiological information regarding drug-induced liver injury in some Latin American countries remains limited. Therefore, disease prevention and health promotion strategies are imperative to reduce drug-induced liver injuries and its fatal outcomes. This study aimed to collect epidemiological data regarding drug-induced liver injury and identify associated factors in patients admitted to a university hospital in Colombia.
Methods and patientsA prospective study was conducted for 1 year to assess the incidence of drug-induced liver injury in patients aged >18 years who showed elevated values in liver tests. Data were collected after obtaining informed consent from the patients. The updated Roussel Uclaf Causality Assessment Method was applied to assess the causality of drug-induced liver injury.
ResultsThe study included 286 patients with elevated values in liver tests, 18 of whom presented with drug-induced liver injury. The mean age of patients was 54.7±19.1 years. The associated pharmacological groups were anti-infectives and anticonvulsants (isoniazid, rifampicin, nitrofurantoin, phenytoin, and valproic acid), with a total of 15 drugs. The affected patients presented with cytopenia, jaundice, nausea, vomiting, or hepatomegaly. The most common type of liver injury was hepatocellular, and most patients recovered satisfactorily. The number of patients who had highly probable and probable causality grading was 1 and 9, respectively.
ConclusionThe incidence of drug-induced liver injury in a university hospital in Colombia was 6%. Comorbidities and concomitant drugs are risk factors for drug-induced liver injury.
Trial registrationRegistered in The Cuban Public Registry of Clinical Trials (identifier RPCEC00000242).
Drug-induced liver injury (DILI) may be a liver damage caused by drug exposure. DILI may be an uncommon yet serious adverse drug reaction, and it is the most common cause of drug withdrawal from the pharmaceutical market [1]. Liver injury is defined by increased serum activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) and may be manifested by a hepatocellular, cholestatic, and mixed pattern [2–4].
The incidence of DILI is variable [5]. Some risk factors that increase the probability of DILI incidence include idiosyncrasy, age, sex, alcohol consumption, concomitant drug usage, and current or past medical history of liver disease as well as genetic and environmental factors [6,7]. Some clinicopathological and histological manifestations of DILI include acute and chronic hepatitis, fulminant hepatitis, cholestasis, or steatosis [8], accompanied by nonspecific symptoms, such as abdominal pain, jaundice, fever, nausea, vomiting, diarrhea, pruritus, and rash. Therefore, the identification of DILI is a complex process; consequently, clinical scales, such as the Roussel Uclaf Causality Assessment Method (RUCAM), have been developed to assess causality [9]. In addition, DILI has no specific treatment and generally includes suspending the suspected drug, treating symptoms, avoiding other possible hepatotoxic agents, and monitoring liver tests [10,11].
Approximately 1100 drugs, excluding substances of abuse and herbal products, have been shown to be associated with DILI [12]. Although most lipophilic drugs likely cause liver impairment, the most commonly associated pharmacological groups include antibiotics (amoxicillin/clavulanic acid or rifampicin), non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac and ibuprofen, antidepressants (paroxetine), and anticonvulsants (phenytoin, carbamazepine, or valproic acid) [13–16]. A recent study has also shown that among intravenous drugs, antibiotics and antineoplastic agents are the pharmacological groups that are most commonly associated with DILI [17]. In a structured review [18], 181 drugs and 17 combined pharmaceutical forms or therapeutic regimens were suspected to induce hepatotoxicity, with eight drugs showing a definite probability (methotrexate, minocycline, vancomycin, everolimus, isoniazid, rifampicin, pyrazinamide, and tamoxifen) and 61 and 119 drugs, respectively, showing probable and possible ability to induce liver injury.
DILI accounts for 1/600 to 1/3500 of all hospital admissions, with 2%–3% hospitalizations for jaundice, 10% for acute hepatitis (>40% in patients aged >50 years), and 15%–30% for fulminant hepatic failure [19,20]. In the United States, France, Switzerland, and Spain, studies have estimated the incidence of DILI [14,16,21]; unfortunately, limited evidence is available regarding its incidence in some Latin American countries, such as Colombia [15], and published information is generally based on clinical case reports. Consequently, the purpose of this study was to collect 1-year epidemiological data regarding DILI and identify its associated factors in patients who were admitted to a university hospital in Colombia.
2Methods and patientsA prospective study was conducted for 1 year (between November 03, 2015, and November 3, 2016) to determine the incidence of DILI in patients who were admitted to a university hospital in Medellin, Colombia. The study was registered in The Cuban Public Registry of Clinical Trials (identifier RPCEC00000242).
Inclusion criteria:
- •
Patients aged >18 years.
- •
Presumptive identification of DILI: patients showing increased values of ALT greater than three times and/or increased values of ALP greater than two times the ULN; hospital stay duration of more than 48h for the follow-up of altered values, allowing for the confirmation of possible DILI.
- •
Patients or caregivers who authorized their participation in the study by signing informed consent forms.
Exclusion criteria:
- •
Patients with increased values of ALT greater than three times the ULN and/or increased values of ALP levels greater than two times the ULN for >6 months.
- •
Patients with acute coronary syndrome.
- •
Patients with terminal disease.
- •
Patients undergoing treatment for some type of neoplasm.
- •
Patients with intoxication.
- •
Patients with alcohol consumption of >20g/day in females and >40g/day in males.
- •
Isolation or death of patients.
The threshold ALT criteria greater than three times for inclusion of patients was used due to medical protocols of the university hospital, this was endorsed by the Bioethics Committee of the institution.
Data were collected by the review of medical records; interview with the patient, caregiver, or health professionals; and bibliographic review of databases. Data were collected using spreadsheets in Microsoft Excel® and processed using Statistical Package for the Social Sciences v23. For quantitative variables, measures of central tendency were presented, and for categorical variables, contingency tables were used to calculate frequency measurements in addition to Chi-squared and Fisher's exact tests. p<0.05 was considered statistically significant.
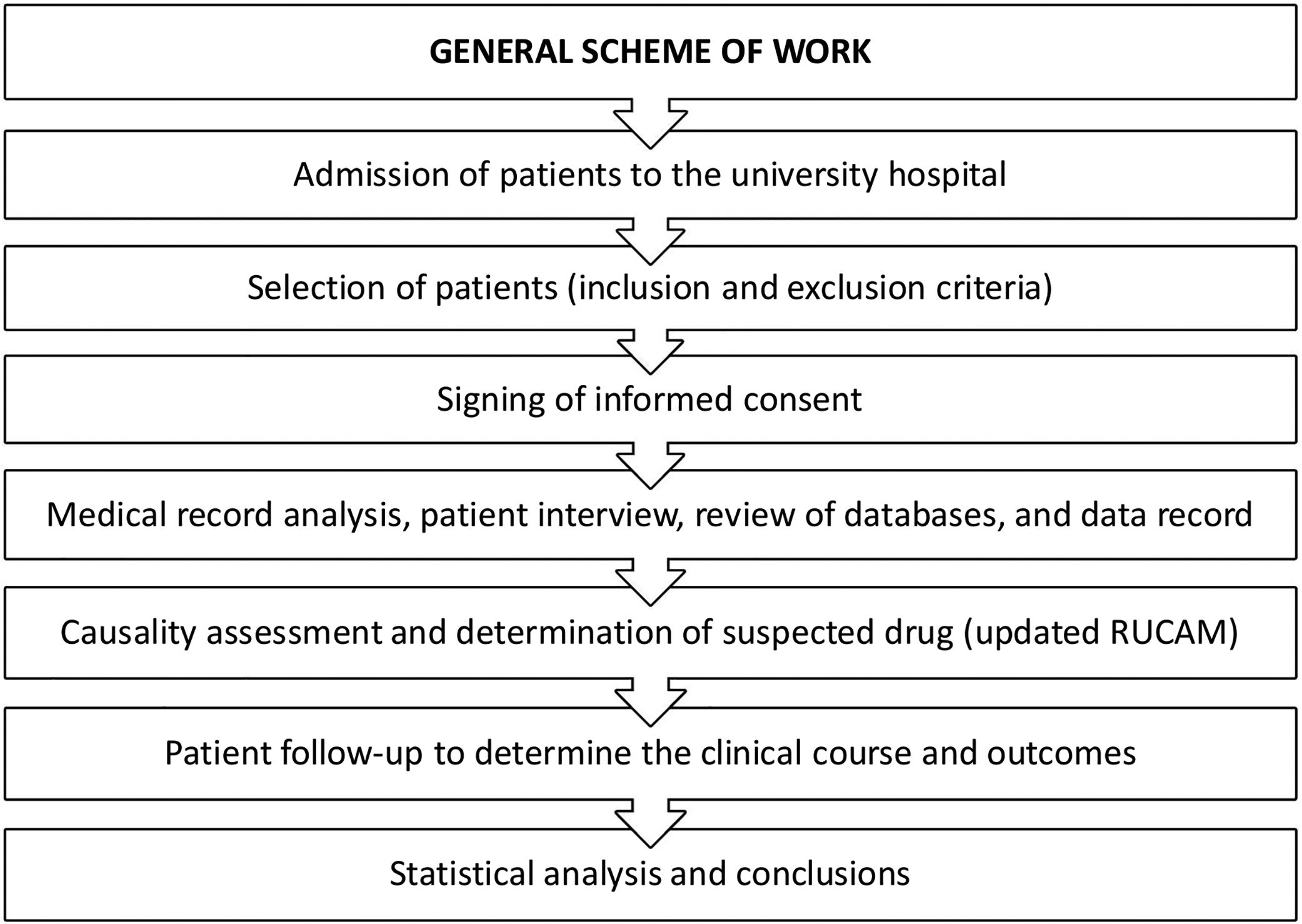
The causality assessment method of DILI was the updated RUCAM [2]. Fig. 1 presents the general scheme of work in this prospective study.
This study was conducted in accordance with the ethical principles of human experimentation established in Colombia and the Declaration of Helsinki. The study was approved by the Bioethics and Investigation Committee of IPS Universitaria Clínica León XIII on July 29, 2015. Voluntary written informed consent was provided by all patients.
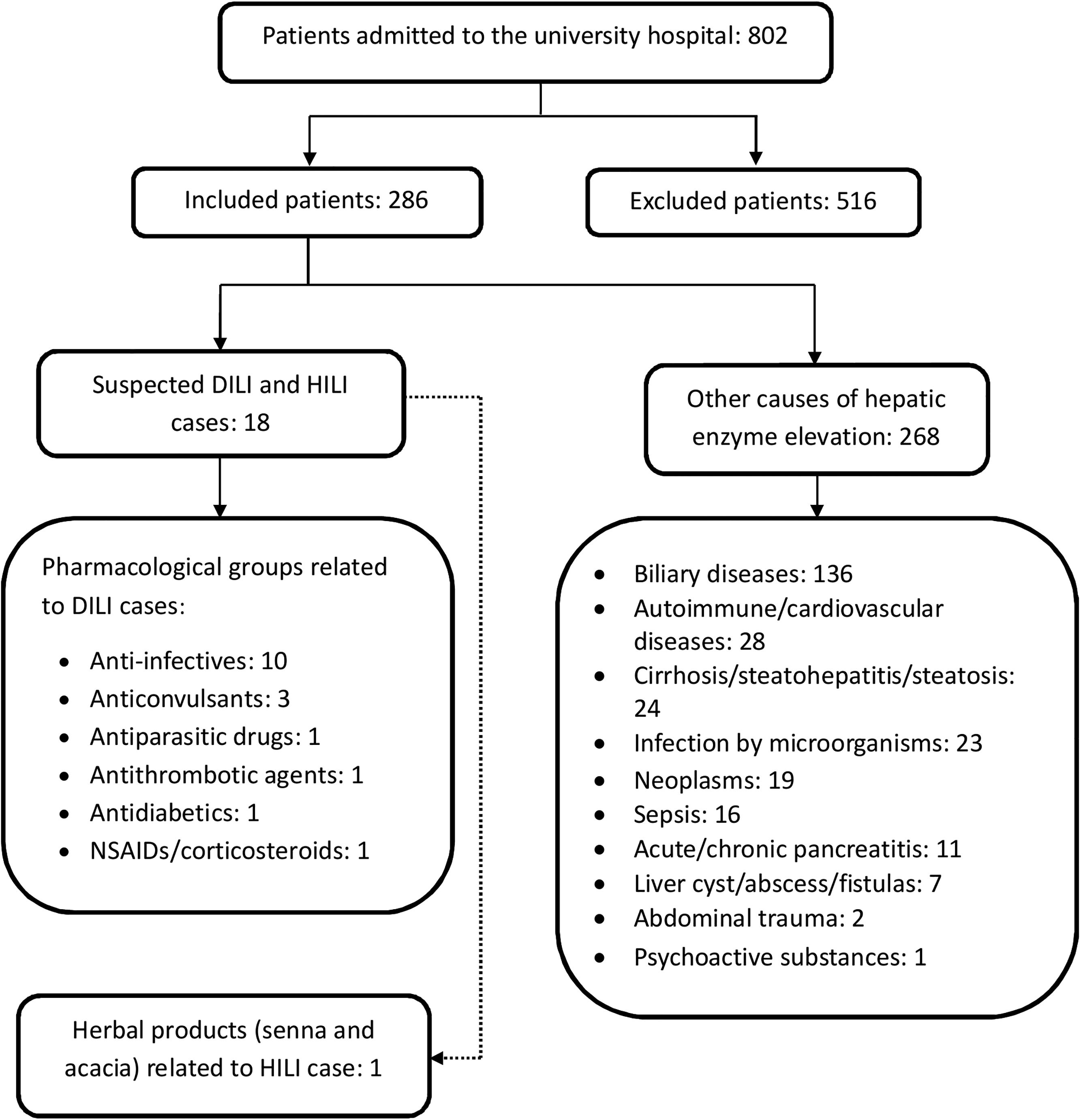
3ResultsAltered values of liver tests (ALT and/or ALP) were detected in 802 patients admitted to the university hospital. The number of excluded patients was 516. In total, 286 patients met the inclusion criteria and had a mean age of 58.0 years (SD, 19.4), of whom 164 patients (57.3%) were female (Fig. 2).
Cases of DILI (n=18): In total, 17 cases of DILI and one case of herb-induced liver injury (HILI) were identified. The incidence of DILI was established during 1 year. For patients who signed the informed consent form, an incidence of 6.29% was obtained, corresponding to 5.94% and 0.35% for drugs and phytotherapeutics, respectively. Moreover, 15 medications (17 active ingredients) and a combination of herbal products associated with DILI and HILI were identified. The mean age of patients was 54.7 years (SD, 19.1), with a female predominance (61.1%).
The risk factors associated with the cases of DILI were concomitant use of medications, such as methotrexate, atorvastatin, leflunomide, itraconazole, or metronidazole (88.9%); comorbidities, such as rheumatoid arthritis, heart failure, histoplasmosis, and liver abscess (33.3%); alcohol consumption not considered as alcoholism (16.7%); previous alcohol consumption (11.1%); and viral infections (11.1%). The nonspecific signs and symptoms presented by patients with DILI included cytopenia, hepatomegaly, jaundice, epigastric pain, vomiting, nausea, fever, choluria, asthenia, rash, eosinophilia >6%, right upper quadrant pain, pruritus, anorexia, acholia, or arthralgia (from higher frequency to lower). One patient was asymptomatic. Female sex could be associated with the presence of jaundice in cases of DILI (p=0.0498).
Pharmacological groups and drugs associated with DILI: Pharmacological groups and drugs were identified using the Anatomical, Therapeutic, Chemical classification system (ATC): anti-infectives [isoniazid, rifampicin, pyrazinamide, ethambutol, trimethoprim/sulfamethoxazole, daptomycin, and nitrofurantoin (50.0%)]; anticonvulsants [phenytoin, valproic acid, and pregabalin (16.7%)]; antiparasitic drugs [albendazole and meglumine antimonate (11.1%)]; NSAIDs [diclofenac (5.6%)]; antithrombotic agents [acetylsalicylic acid (5.6%)]; antidiabetics [metformin (5.6%)]; and corticosteroids [dexamethasone (5.6%)].
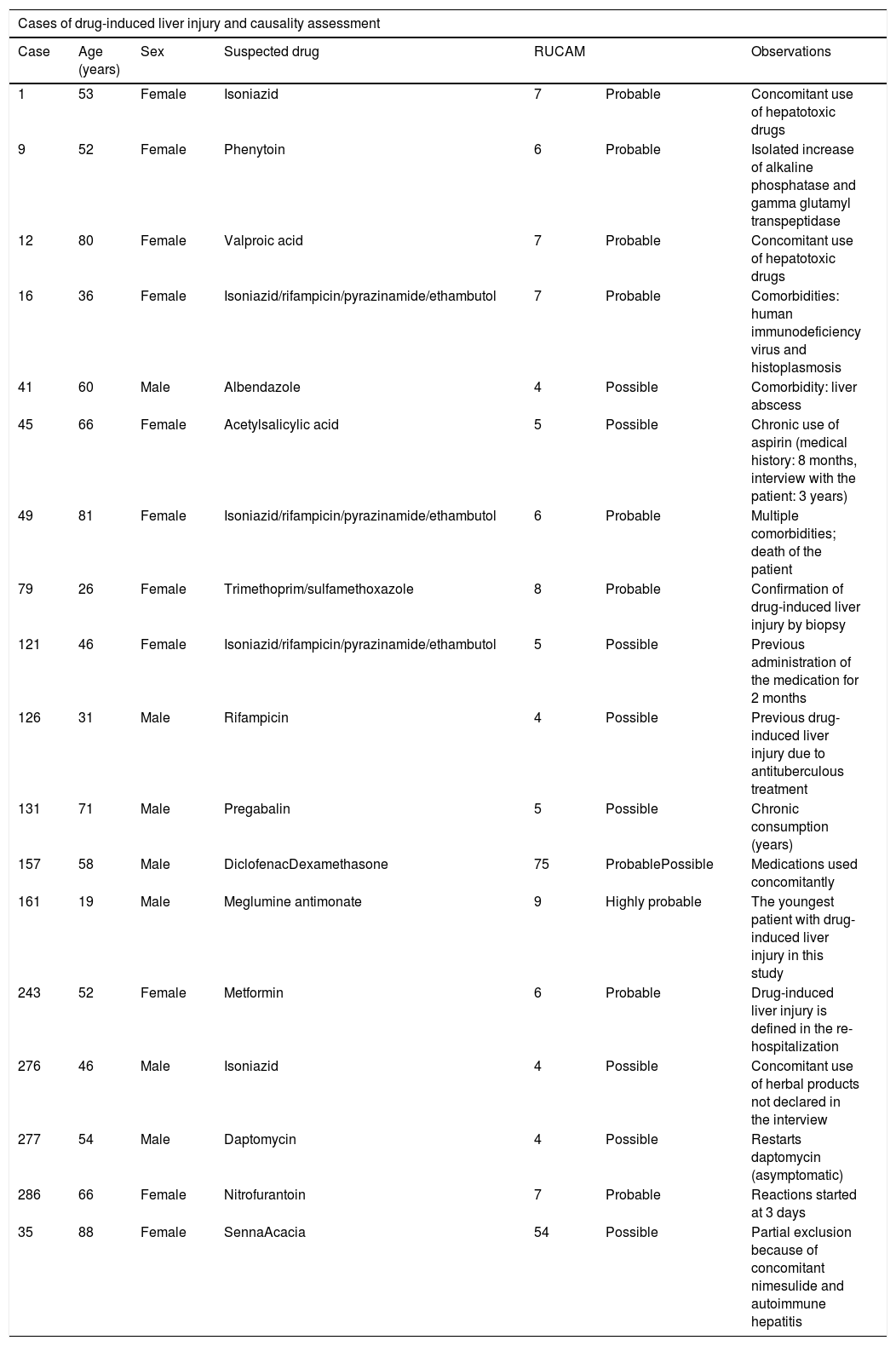
To establish the causality of DILI, the RUCAM scale was used. The number of patients who had highly probable and probable causality grading was 1 and 9, respectively. Table 1 summarizes the cases and causality assessment.
Cases of drug-induced liver injury and causality assessment.
| Cases of drug-induced liver injury and causality assessment | ||||||
|---|---|---|---|---|---|---|
| Case | Age (years) | Sex | Suspected drug | RUCAM | Observations | |
| 1 | 53 | Female | Isoniazid | 7 | Probable | Concomitant use of hepatotoxic drugs |
| 9 | 52 | Female | Phenytoin | 6 | Probable | Isolated increase of alkaline phosphatase and gamma glutamyl transpeptidase |
| 12 | 80 | Female | Valproic acid | 7 | Probable | Concomitant use of hepatotoxic drugs |
| 16 | 36 | Female | Isoniazid/rifampicin/pyrazinamide/ethambutol | 7 | Probable | Comorbidities: human immunodeficiency virus and histoplasmosis |
| 41 | 60 | Male | Albendazole | 4 | Possible | Comorbidity: liver abscess |
| 45 | 66 | Female | Acetylsalicylic acid | 5 | Possible | Chronic use of aspirin (medical history: 8 months, interview with the patient: 3 years) |
| 49 | 81 | Female | Isoniazid/rifampicin/pyrazinamide/ethambutol | 6 | Probable | Multiple comorbidities; death of the patient |
| 79 | 26 | Female | Trimethoprim/sulfamethoxazole | 8 | Probable | Confirmation of drug-induced liver injury by biopsy |
| 121 | 46 | Female | Isoniazid/rifampicin/pyrazinamide/ethambutol | 5 | Possible | Previous administration of the medication for 2 months |
| 126 | 31 | Male | Rifampicin | 4 | Possible | Previous drug-induced liver injury due to antituberculous treatment |
| 131 | 71 | Male | Pregabalin | 5 | Possible | Chronic consumption (years) |
| 157 | 58 | Male | DiclofenacDexamethasone | 75 | ProbablePossible | Medications used concomitantly |
| 161 | 19 | Male | Meglumine antimonate | 9 | Highly probable | The youngest patient with drug-induced liver injury in this study |
| 243 | 52 | Female | Metformin | 6 | Probable | Drug-induced liver injury is defined in the re-hospitalization |
| 276 | 46 | Male | Isoniazid | 4 | Possible | Concomitant use of herbal products not declared in the interview |
| 277 | 54 | Male | Daptomycin | 4 | Possible | Restarts daptomycin (asymptomatic) |
| 286 | 66 | Female | Nitrofurantoin | 7 | Probable | Reactions started at 3 days |
| 35 | 88 | Female | SennaAcacia | 54 | Possible | Partial exclusion because of concomitant nimesulide and autoimmune hepatitis |
RUCAM: Roussel Uclaf Causality Assessment Method.
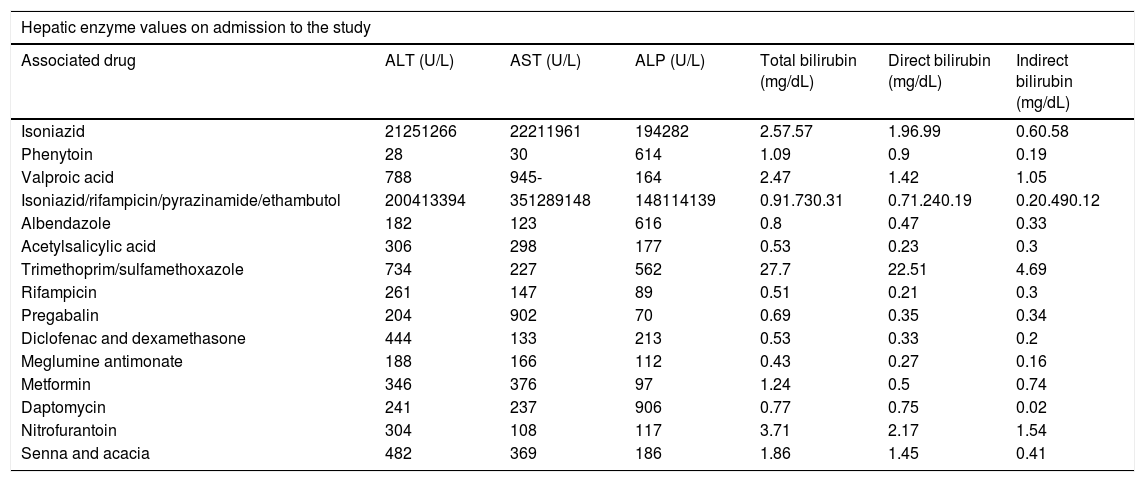
Alteration in liver tests and types of liver injury: Inclusion in the study was determined on the basis of alterations in ALT, corresponding to a value of >147IU/L, and alterations in ALP, equivalent to a value of >258IU/L. The values were determined according to the standard ranges used in the laboratory of the university hospital. Table 2 summarizes the values of patients on the day of admission to the study. Hepatocellular lesion was the most predominant type of injury (72.2%), caused by the use of antituberculous treatment, nitrofurantoin, meglumine antimonate, valproic acid, pregabalin, acetylsalicylic acid, metformin, diclofenac, or dexamethasone as well as the combination of two herbal products (senna and acacia, common names of these plants in the local context). Cholestatic lesion was the second most common type of injury (22.2%), caused by the use of albendazole, daptomycin, trimethoprim/sulfamethoxazole, or phenytoin. Mixed lesion was caused by the use of isoniazid (5.6%).
Values in liver tests.
| Hepatic enzyme values on admission to the study | ||||||
|---|---|---|---|---|---|---|
| Associated drug | ALT (U/L) | AST (U/L) | ALP (U/L) | Total bilirubin (mg/dL) | Direct bilirubin (mg/dL) | Indirect bilirubin (mg/dL) |
| Isoniazid | 21251266 | 22211961 | 194282 | 2.57.57 | 1.96.99 | 0.60.58 |
| Phenytoin | 28 | 30 | 614 | 1.09 | 0.9 | 0.19 |
| Valproic acid | 788 | 945- | 164 | 2.47 | 1.42 | 1.05 |
| Isoniazid/rifampicin/pyrazinamide/ethambutol | 200413394 | 351289148 | 148114139 | 0.91.730.31 | 0.71.240.19 | 0.20.490.12 |
| Albendazole | 182 | 123 | 616 | 0.8 | 0.47 | 0.33 |
| Acetylsalicylic acid | 306 | 298 | 177 | 0.53 | 0.23 | 0.3 |
| Trimethoprim/sulfamethoxazole | 734 | 227 | 562 | 27.7 | 22.51 | 4.69 |
| Rifampicin | 261 | 147 | 89 | 0.51 | 0.21 | 0.3 |
| Pregabalin | 204 | 902 | 70 | 0.69 | 0.35 | 0.34 |
| Diclofenac and dexamethasone | 444 | 133 | 213 | 0.53 | 0.33 | 0.2 |
| Meglumine antimonate | 188 | 166 | 112 | 0.43 | 0.27 | 0.16 |
| Metformin | 346 | 376 | 97 | 1.24 | 0.5 | 0.74 |
| Daptomycin | 241 | 237 | 906 | 0.77 | 0.75 | 0.02 |
| Nitrofurantoin | 304 | 108 | 117 | 3.71 | 2.17 | 1.54 |
| Senna and acacia | 482 | 369 | 186 | 1.86 | 1.45 | 0.41 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase.
Liver biopsies were performed in patients with toxicity caused by the use of trimethoprim/sulfamethoxazole (acute hepatitis with necrosis around the central vein and cholestasis), metformin (cytoplasmic cholestasis, chronic cholangitis), senna and acacia (chronic hepatitis with focal necrosis), and isoniazid (chronic hepatitis with cytoplasmic cholestasis). In other patients, skin, kidney, or stomach biopsies were performed for differential diagnosis.
Patient management and reaction outcomes: The suspension of the suspected drug was the first measure taken to improve the reaction outcomes of patients with DILI. In three patients treated with antituberculous treatment and anticonvulsants, changing the pharmacotherapy of each patient was necessary. In 11 patients, using other medications, such as N-acetylcysteine, prednisolone, prednisone, diphenhydramine, hyoscine butylbromide, dipyrone, metoclopramide, or topical betamethasone, was necessary for treating reactions.
In total, 16 patients showed favorable clinical course with improvements in symptoms and liver tests, although in some patients, the values of ALT or ALP did not decrease to less than two times the ULN before discharge. One patient died because of advanced age and multiple comorbidities. One patient was referred to another hospital because of unstable condition. No patient required liver transplant during the hospital stay.
4DiscussionProspective studies like ours allow the determination of the suspected drug in DILI cases; moreover, clinical features can be established and causality can be assessed because of follow-up in real time [22]. Prospective studies are important when the patient values in liver tests do not decrease to normality immediately, as was observed in some of the cases in the present study and in others in the prospective study conducted by Rathi et al. They found that the laboratory variables at 1 week predicted mortality better than those at the initial recognition of the liver injury [23]. Prospective studies like ours provide an opportunity to study a target population and help in the prevention of serious and potentially fatal outcomes [24,25]. Epidemiological information regarding the frequency of the use of hepatotoxic drugs was established in the present prospective study, similar to the study conducted by Licata et al. [26]. Prospective analysis allows the determination of the use of other substances (one patient with HILI was determined in the present study). However, further research is necessary in this population regarding the use of herbal products and its consequences; as the increasing incidence of complementary and alternative medicines-induced liver injury reported by Hillman et al. [27].
The incidence of DILI varies depending on the type of study. Bell et al. have reviewed the incidence through prospective studies, such as a study by Ostapowicz (2002) in the United States, which reported an incidence of 13.0%, another by Larson (2005) in the United States, which reported an incidence of 12.0%, and another by Sgro in France, which reported an incidence of approximately 14%. However, these incidences were determined in studies with more than 1 year of follow-up in a larger number of patients than in the present study. Nonetheless, the incidence obtained in the present study is similar to that reported in some retrospective studies that included a larger number of patients and greater duration than those in our study: a study by Valle (2006) in Sweden reported an incidence of 6.6%, that by Hussaini (2007) in England reported an incidence of 8.1%, and finally, that by Russo (2004) in the United States reported an incidence of 6.0%. Despite the limited information available regarding DILI in Colombia, our study provides an estimate of liver injury caused by the use of medications. Regarding phytotherapeutics, many studies have focused on traditional Chinese medicine, with incidences ranging 4%–7% [2]. In addition, case reports regarding HILI (including green tea) have been published in Colombia and Spain [28–30].
Alternative causes of liver disease improve the identification of DILI. In the present study, the main alternative causes of increases in values in liver tests included the following: vesicular lithiasis, cholangitis, cholecystitis, cirrhosis, pancreatitis, infection by microorganisms, neoplasms, as well as autoimmune and cardiovascular diseases. The most common alternative causes reported are biliary diseases, autoimmune or ischemic hepatitis, systemic sepsis, and viral infections [31–34]. The neoplasms were previously reported [34] as causes of elevation in hepatic enzymes; in the present study, 19 patients with neoplasms were detected. Furthermore, alcoholism and intoxication have been reported in the literature as alternative causes; however, in the present study, these formed a part of the exclusion criteria. Additionally, alterations in liver tests may be due to unknown reasons [33], for example, one patient in our study was determined as not having DILI by the physician, because his clinical features din not fit in the previously reported alternative causes, the reason remained unknown. In the present study, the pharmacist's intervention in the interview with the patient, optimized the causality assessment of DILI by obtaining extra information and using the RUCAM prospectively.
Hepatotoxic drugs detected in the present study are consistent with the information available in the literature. The main pharmacological groups were antibiotics, NSAIDs, antidepressants, and anticonvulsants [13–16,26]. Although case reports have discussed the association of antineoplastic agents with DILI [7–13], our study did not detect any such association. Furthermore, the risk factors in the present study are consistent with those reported in previously published studies [7,20,35]; age is an important risk factor as well as comorbidities, polymedication, and concomitant use of hepatotoxic drugs. Although the female sex is commonly affected by medications [36], the incidence of DILI was not associated with sex in our study.
Previous studies on DILI have reported nonspecific signs and symptoms in patients [15,37,38], which is similar to the observation in our study. The presence of jaundice could be associated with female sex, but further research is imperative to establish such an association. Most of the patients admitted in the present study presented with increased values of ALT, indicating that the predominant type of liver injury in this study was hepatocellular lesion, followed by cholestatic lesion, whereas mixed lesion was observed in only one patient; this was similar to the tendency reported in other studies [14,26]. Cholestasis detected in the liver biopsy of a patient receiving trimethoprim/sulfamethoxazole is consistent with the results reported previously [24,39,40]. Similarly, findings of the liver biopsies of patients receiving metformin and isoniazid in the present study are also consistent with those reported previously [1,41–44].
Regarding the scale used to assess the incidence of DILI, RUCAM has shown certainty and usefulness in clinical practice [9]. This scale facilitates the identification of the causative agent when concomitant drugs are administered because the parameters can be analyzed with greater precision.
In clinical practice, N-acetylcysteine has traditionally been used as an antidote for acetaminophen toxicity, and considering that there are no specific antidotes for DILI, the physicians used N-acetylcysteine to improve the condition of patients, particularly in patients with liver injury due to antituberculous medications, in the present study [45–47]. Nonetheless, future studies are warranted to analyze the use of N-acetylcysteine as a premedication to prevent liver injury in patients undergoing hepatotoxic treatments. The use of corticosteroids, such as prednisone, prednisolone, and betamethasone, to improve symptoms, was adjusted to previously published recommendations [11]. Therefore, these measures could be posited to have facilitated the recovery of most of the cases presented in our study.
In summary, the 1-year incidence of DILI in a university hospital in Colombia was approximately 6%. The main pharmacological groups associated with DILI were anti-infectives and anticonvulsants. Furthermore, the presence of comorbidities and the use of concomitant drugs were important risk factors in Colombian patients. We recommend that the suspension of the suspected drug must be the first protective measure implemented, followed by the change of pharmacotherapy or administration of medications to improve the condition of patients, as required.
Limitation of the study: This study was conducted over a short period of time and in a single hospital. One of the reasons for the loss of patients was the isolation of patients per rules of the hospital, which did not allow the signing of informed consent. The usual threshold ALT criteria for inclusion of patients could not be used due to medical protocols of the university hospital, this was endorsed by the Bioethics Committee of the institution. Some patients had a RUCAM-based causality gradings of possible allowing establish an approximate incidence of DILI, which needs to be confirmed with further studies.AbbreviationsDILI drug-induced liver injury alanine aminotransferase aspartate aminotransferase alkaline phosphatase total bilirubin upper limit of normal Roussel Uclaf Causality Assessment Method number of cases non-steroidal anti-inflammatory drugs herb-induced liver injury Anatomical, Therapeutic, Chemical classification system
The Pharmaceutical Promotion and Prevention Research Group received economical support from “Comité para el Desarrollo de la Investigación (CODI),” Call for sustainability 2018–2019, University of Antioquia, Medellin, Colombia.
Conflict of interestThe authors declare no conflict of interest.
The authors would like to thank the University of Antioquia and the members of the Toxicology and Pharmacovigilance Department of the IPS Universitaria Clínica León XIII.