Ursodeoxycholic acid is the first-line therapy for primary biliary cholangitis. However, a subset of patients fail to show biochemical response. For these patients, adjuvant therapies are warranted. Obeticholic acid was conditionally approved as a second-line drug. Evidence is building up in favor of fibrates, which are available for off-label use.
Primary biliary cholangitis (PBC) is a chronic, immune-mediated liver disease characterized by progressive inflammation and destruction of the small intrahepatic bile ducts, eventually leading to biliary cirrhosis. Both the incidence and prevalence of PBC seem to be increasing, at least in part due to increased awareness among physicians, availability of reliable non-invasive testing (anti-mito-chondrial antibody) and long-term treatment with urso-deoxycholic acid (UDCA). In that regard, use of UDCA at recommended doses of 13-15 mg/kg/day has been shown to delay histological progression, delay development of es-ophageal varices and improve survival free of liver transplantation. In fact, a decline was noted in recent years in the absolute number of patients listed for and transplanted for PBC, although mortality may be increasing in the waiting list due to frailty and malnourishment.1 Thus, despite the above-mentioned beneficial effects of UDCA, a subgroup of patients continue to progress towards end-stage liver disease and either die or need a liver transplant. These “at-risk” patients can be recognized early as they fail to demonstrate a biochemical response to UDCA.
Biochemical response to UDCA should be assessed 1 year after therapy has been initiated. Up to 40% of patients become non-responders and are at risk for progression to end-stage liver disease as well as development of hepato-cellular carcinoma. Younger patients at the time of diagnosis, males and Hispanics are less likely to respond to UDCA and should be monitored closely. While many criteria have been proposed to define non-response, more robust models were developed recently to assess response and provide prognostic information. These include the PBC Globe score (http://www.globalpbc.com/globe) and the UK-PBC score (http://www.uk-pbc.com/resources/ tools/riskcalculator/). For instance, a Globe PBC score > 0.3 indicates a lower probability of survival without a liver transplant compared to age- and gender-matched controls (high risk patient). The model also offers estimated survival at 3, 5, 10 and 15 years and compares that to the general population.
In May 2016 the FDA granted accelerated approval for obeticholic acid (OCA, Ocaliva), a Farnesoid X agonist, as adjuvant therapy to be used together with UDCA for patients with inadequate response to UDCA after at least 1 year of treatment. Patients intolerant to UDCA could use OCA as monotherapy. Approval of OCA was based on well-documented improvements in serum alkaline phosphatase, considered by the FDA a “reasonably likely to predict” surrogate marker, one that is not yet well validated.2 The term “accelerated approval” implies that it is conditional on further studies confirming the clinical benefit of OCA. Although the phase 3 study could not demonstrate an effect on well-defined endpoints such as mortality, need for liver transplantation, improvement of symptoms (such as pruritus) or quality of life. Post-marketing studies are ongoing to address that. Furthermore, modeling studies indicate that addition of OCA to UDCA can reduce the cumulative incidence of decompensated cirrhosis, hepatocellular carcinoma, liver transplantation and liver-related deaths. OCA can be prescribed in the US and Europe at an annual cost of approximately $70,000, which unfortunately, is not a cost-effective strategy. A price reduction in the range of 70% would be necessary to make OCA cost-effective!3
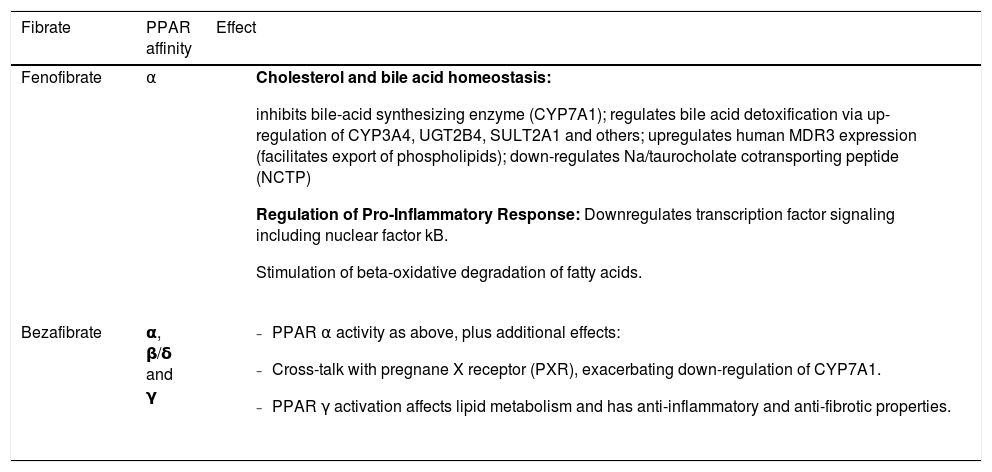
Fibrates, on the other hand, are readily available around the world and are cheap. Fibrates act through a different nuclear receptor, the Peroxisome Proliferator-Activated Receptor (PPAR), which exists in 3 isoforms: a,(3/8 and y, each with variable expression in different organs. PPARcc is mainly expressed in the liver (but also in the muscle, kidney and heart), PPARy in adipose tissues and immune system, and PPAR-(3/8 is ubiquitous. Upon activation, PPARs regulate gene transcription by forming a het-erodimer with retinoid X receptor and binding to its specific DNA response elements. Each fibrate has different specificity for the various PPAR subtypes, which in turn translates into a different effect, as shown in table 1.4 The main mechanism of action of fibrates in PBC is through upregulation of genes involved in biliary phospholipid secretion, while downregulating synthesis of bile acids and downregulating the NF-kB inflammatory pathway. Fibrates can stimulate transcription and canalicular insertion of multidrug resistance protein 3 (MDR3). With increased secretion of phosphatidylcholine, bile becomes less toxic. In PBC, non MDR3-dependent mechanisms also contribute to the anti-cholestatic effects through repression of bile acid synthesis and decreased uptake of bile acids into the hepatocytes.4,5
Mechanisms of anti-cholestatic effects of fibrates according to their affinity for specific PPAR subtypes.
| Fibrate | PPAR affinity | Effect |
|---|---|---|
| Fenofibrate | α |
|
| Bezafibrate | α, β/δ and γ |
|
Several small clinical trials have evaluated the use of fi-brates (fenofibrate, bezafibrate) for the management of PBC, and meta-analyses were conducted to summarize their effect, all indicating a beneficial effect on liver chem-istries.5 Of all available studies, the most impressive is the BEZURSO, a randomized placebo-controlled trial including 100 patients with PBC and incomplete response to UDCA. Patients were randomized to a 2-year treatment period with either bezafibrate 400 mg/day or placebo, in addition to UDCA. Both groups were similar at baseline. The primary endpoint was complete normalization of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, albumin and pro-thrombin time by month 24; this was achieved by 30% of patients on bezafibrate and none on the placebo group. Notably, 67% of patients on bezafibrate normalized alkaline phosphatase, compared to 0% on placebo, and the drug was very well tolerated.6 Other important endpoints such as liver stiffness by transient elastography and the itch score significantly improved in the bezafibrate group compared to placebo, indicating perhaps that this drug can halt disease progression AND improve how the patient feels, both important efficacy measures. Another large, long-term study with bezafibrate for itching in PBC is currently ongoing in the Netherlands (http://clinicaltrials.gov/show/NCT02701166). While use of fibrates, like that of OCA, has not yet led to demonstration of improved survival, the reported improvement in pruritus is also a clinically useful endpoint as it affects the way our patients live with their disease. The community of experts and patients is hopeful that the Dutch study will corroborate those findings. Fibrates are overall very well tolerated, with minor side effects of heartburn, myalgias and transient transaminase elevations reported in clinical trials for PBC. Concerns over increased risk of kidney dysfunction and gallstone formation have not materialized, but rare cases of hepatotoxicity have been published in non-PBC patients.
While we await post-marketing studies to confirm a beneficial effect of OCA, clinicians have the option of using OCA, where available, or an off-label medication approved only for the treatment of dyslipidemia and which has also produced remarkable improvement in liver biochemistries in patients with PBC - the same liver biochemistries previously validated as reliable and robust prognostic markers by the Global PBC study group (ALP and TB).7