Fibrosis accompanies most chronic liver disorders and is a major factor contributing to hepatic failure. Therefore, the need for an effective treatment with the aim of modifying the clinical course of this disease is evident. The aim of this work is to determine whether genistein, which has been shown to modulate the physiology and pathophysiology of liver, is able to decrease experimental liver fibrosis and cholestasis. In male Wistar rats, the common bile duct was ligated. Administration of genistein (5 μg rat-1, day-1, p.o.) began four weeks after biliary obstruction and continued for a further four weeks. The liver was used for histological and ultrastructural analysis and for collagen quantification (hydroxyproline content). The degradation of Matrigel® and collagen type I was determined in homogenized liver. Bilirubins and enzyme activities were measured in serum. Genistein was able to improve normal liver histology, ultrastructure, collagen content, and biochemical markers of liver damage. It also increased Matrigel® and collagen type I degradation. In summary, the present report shows that genistein inhibits the fibrosis and cholestasis induced by prolonged biliary obstruction in the rat. Genistein has therapeutic potential against liver fibrosis.
Abbreviations:
ECM: extracellular matriz, NASH: non-alcoholic steatohepatitis, HSC: hepatic stellate cells, SMA: smooth muscle α-actin, ROS: reactive oxygen species, TPK: protein tyrosine kinase, TGF-β 1: transforming growth factor,
IntroductionLiver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix proteins, which is a characteristic of most types of chronic liver diseases.1-4 The main causes of liver fibrosis in industrialized countries include chronic HCV infection, alcohol abuse, and nonalcoholic steatohepatitis (NASH). The accumulation of ECM proteins distorts the hepatic architecture by forming a fibrous scar, and the subsequent development of nodules of regenerating hepatocytes defines cirrhosis.5,6
Hepatic fibrosis is considered a model of wound-healing response to chronic liver injury, and it is characterized by the activation of hepatic stellate cells (HSCs). The activation of HSCs involves the transdifferentiation from a quiescent state into myofibroblast-like cells with the appearance of smooth muscle a-actin (SMA) and loss of cellular vitamin A storage.7 The activated HSCs are distinguished by accelerated proliferation and enhanced production of ECM components. Cross-talks between parenchymal and nonparenchymal cells constitute the major interactions in the development of hepatic injury and fibrosis. Soluble factors, such as cytokines, chemokines, or reactive oxygen species are the mediators in these cross-talks, and are possible targets for therapeutic consideration8].
Genistein (4,5,7-trihydroxyisoflavone), a soy-derived isoflavone, has recently attracted much attention in the medical scientific community. Genistein is a potent protein tyrosine kinase inhibitor that attenuates growth factor-and cytokine-stimulated proliferation of both normal and cancer cells.10 Extensive epidemiological, in vitro, and animal studies have been performed, and most studies indicated that genistein has beneficial effects on a multitude of human disorders, including cancers, cardiovascular diseases, osteoporosis, and postmenopausal symptoms.11,13 The role of genistein in the physiology and pathophysiology of liver has been studied in the last decade.14-20 More than a dozen reports regarding the effect of genistein on HSCs have appeared. Antifibrotic effects of genistein in vitro have been shown.21,23 However, at present there are no reports regarding the effect of genistein on fibrogenesis in vivo. Therefore, the aim of this study was to evaluate the effect of genistein on the fibrosis and cholestasis induced by prolonged biliary obstruction in the rat.
Materials and methodsAnimal treatment and biliary obstructionMale Wistar rats weighing 200 g were used. Animals had free access to food (Standard Purina Chow; St Louis, MO) and water. Obstructive jaundice was induced by double ligation and sectioning of the common bile duct. Control rats were sham operated. Genistein (Sigma Chemical Co., St Louis, MO) was dissolved in water and administered at a dose of 5μg per rat through an intragastric tube. This chosen dose was based on previous studies by our group.24 Administration of genistein began four weeks after biliary obstruction and was continued for a further four weeks. Another group of animals was bile duct ligated, but received only water, instead of the drug (fibrosis group). Each group consisted of six rats. The animals were sacrificed eight weeks after surgery under light ether anesthesia; blood was collected by heart puncture, and the liver was rapidly removed. Small liver sections fixed in Bouin’s medium were used for trichromic staining for histological examination under light microscopy. This investigation followed the Guiding Principles in the Care and Use of Animals from the National Institutes of Health 25.
Collagen quantificationCollagen concentration was determined by measuring hydroxyproline content in fresh liver samples after digestion with acid.26 The procedure was as follows. Fresh liver samples (100 mg) were placed in ampoules, 2 ml of 6 N HCl was added, and the ampoules were sealed and hydrolyzed at 100 °C for 48 h. The samples were then evaporated at 50 °C for 24 h and resuspended in 3 ml of sodium acetate/citric acid buffer (pH 6.0); 0.5 g of activated charcoal was added, and the mixture was stirred vigorously then centrifuged at 5000 xg for 10 min. The mixture was kept for 20 min at room temperature and the reaction was stopped by the addition of 2 M sodium thiosulfate and 1 N sodium hydroxide. The aqueous layer was transferred to test tubes. The oxidation product from hydroxyproline was converted to pyrrole by boiling the samples. The pyrrole-containing samples were incubated with Ehrlich’s reagent for 30 min and their absorbance was read at 560 nm. Recovery of known amounts of standards was carried out on similar liver samples to provide calibration samples.
Tissue extraction of proteasesLiver samples were homogenized with 10 volumes of buffer (0.075 M) potassium acetate, 0.3 M NaCl, 0.01 M EDTA, 0.1 M L-arginine and 0.25% Triton X-100; pH 4.2) on ice. After being kept in ice water for 3 h, the homogenized samples were centrifuged at 12 000 xg and 4 °C for 10 min. Supernatants were stored at -70 °C until assay.
Proteolytic activity assaysAssay plates were prepared by diluting ice-cold collagen I in 0.2% acetic acid with an equal volume of neutralizing buffer (100 mM Tris-HCl, 200 mM NaCl, 0.04% NaN3; pH 7.8) to give a final collagen concentration of 700 μg/mL. Aliquots (50 ¡uL) of the diluted collagen were added quickly to microwell modules and incubated at 30 °C for 40 h (16 h in a humidified atmosphere, then 24 h in a dried atmosphere) to gel and dry the collagen. The same procedure was performed using Matrigel® basement membrane extract. Aliquots of 100 μg were used per well and dried overnight after polymerization at room temperature.
The enzymatic essay was performed as described by Nethery et al.27 Enzyme samples were brought to assay temperature (35 °C) and replicate aliquots (100 μL) were added to the protein-coated wells. Microwells containing samples were incubated for 2 h in a humidified container equilibrated at assay temperature. The samples were decanted from the wells, which were washed twice in quick succession with assay buffer (50 mM Tris-HCl, 100 mM NaCl, 10mM CaCl2, 0.02% NaN3; pH 7.5). Stain solution (100 uL of 0.25 g Coomassie Blue R250, 10% acetic acid, 50% methanol) was incubated in the wells for 25 min at 25 °C. After the stain was drained off, the wells were washed three times with distilled water and left to dry at room temperature. The absorbance at 590 nm was measured using a Titertek Multiscan automatic spectro-photometer (Flow Laboratories, Titertek, Huntsville, AL) with the instrument blank set on unused microwells. Collagenase and urokinase were used as standard samples.
Serum enzyme activities and bilirubinsSerum was obtained for the following determinations: the activities of alkaline phosphatase, alanine amino transferase, μ-glutamyl transpeptidase, and for bilirubin content (Kit Merck, México)
Electron microscopic analysisFor ultrastructural analysis, liver blocks of ca 0.5-0.7 mm3 were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 1 h and 1% OsO4 was added to continue fixing (30 min at 27 °C and 30 mi4n at 4 °C). After dehydration with increasing concentrations of ethyl alcohol, the blocks were embedded in epoxy resin. Ultrathin sections (80 nm) were cut and then examined with a Jeol-200 EX electron microscope at an accelerating voltage of 80 kV.
Statistical analysisData are reported as means ± standard deviation of three independent experiments conducted in quadruplicate. Statistical analysis was performed using a non parametric ANOVA. Individual differences between treatments was analyzed by a Tukey’s test. Significant differences were established at p < 0.001.
ResultsFigure 1 shows the histological analysis of liver sections. Prolonged biliary obstruction was accompanied by an increase in collagen deposition around the portal triad (Figure 1C). In addition, the normal architecture was lost and a marked ductular proliferation was observed. Genistein treatment for four weeks in bile duct-ligated rats restored the normal architecture of the liver. A significant decrease in the collagen content was observed in the bile duct-ligated rats treated with genistein (Figure ID).
Histological study of liver sections from rats treated with genistein. Liver sections from: (A) Control (Sham operated) rats; (B) Genistein; (C) bile duct-ligated rats; (D) bile duct-ligated rats and treated with genistein. Liver tissue were stained with Masson trichrome, collagen can be recognized by blue staining (200 X).
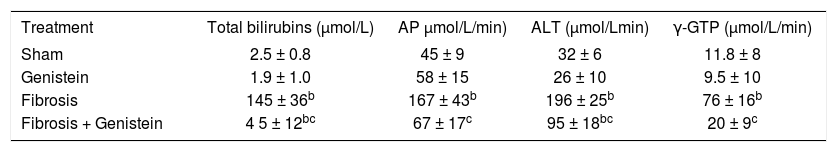
Serum markers of cholestasis and liver injury are shown in Table I. Total bilirubins increased more than 50-fold compared with controls. Serum activities of alkaline phosphatase, alanine aminotransferase, and γ-glutamiltranspeptidase increased approximately three-, six-, and sixfold, respectively, compared to the control group (p < 0.001). Genistein administration to bile duct-ligated rats significantly reduced the markers of cholestasis and liver injury.
Effect of genistein on Total Bilirubins (TB), Serum Alkaline phosphatase (AP), Serum Alanine amino trasferase (ALT) and γ-Glutamil transpeptidase (γ-GTP)a.
Liver collagen content was estimated in liver samples by measuring hydroxyproline. Biliary obstruction induced a sixfold increase in liver collagen content. Treatment of bile duct-ligated animals with genistein significantly decreased the collagen content; p < 0.001 (Figure 2).
Liver collagen content determined in sham-operated rats (sham), genistein treated rats, bile duct-ligated rats (fibrosis) and bile duct-ligated rats and treated with genistein (fibrosis +genistein). Each bar represents the mean ± SEM in experiments performed in duplicate. All groups consisted of six animals. * Means different from the Sham group (p<0.001). # means different from the fibrosis group (p < 0.001).
Degradation of collagen type I is shown in Figure 3. Bile duct ligation increased twofold the degradation of collagen type I; genistein administration to bile duct-ligated rats increased this activity significantly (3.6-fold; p < 0.001). Matrigel® degradation by liver homogenates was used as an indicator of the connective tissue degradation capacity of the liver. Figure 4 show that genistein doubled Matrigel® degradation when administered to bile duct-ligated rats only.
Type I collagen degradation by liver homogenates determined in sham-operated rats (sham), genistein treated rats, bile duct-ligated rats (fibrosis) and bile duct-ligated rats and treated with genistein (fibrosis +genistein). Each bar represents the mean ± SEM in experiments performed in duplicate. All groups consisted of six animals. * Means different from the Sham group (p < 0.001). # means different from the fibrosis group (p < 0.001).
Quantitative Matrigel® degradation by liver homogenates deterHned in sham-operated rats (sham), genistein treated rats, bile duct-ligated rats (fibrosis) and bile duct-ligated rats and treated with genistein (fibrosis +genistein). Each bar represents the mean ± SEM in experiments performed in duplicate. All groups consisted of six animals. * Means different from the Sham group (p < 0.001). # means different from the fibrosis group (p < 0.001).
Ultrastructural analysis of the livers is shown in Figure 5. Panel A shows an electron micrograph of a liver section from a control rats (sham) in which hepatocyte are well organized. However, bile duct-ligated rats a high disorganization of hepatocytes with diverse grade of degeneration. We observed the presence of some HSCs activated associated with some collagen fibers (panel C). On the other hand, bile duct-ligated rats treated with genistein restored the ultra structure of hepatocytes which correlated with the improvement of liver function (panel D).
DiscussionHepatic fibrosis is a dynamic process resulting from chronic damage up to cirrhosis, characterized by accumulation of ECM components in the liver, caused by both markedly increased and unbalanced degradation of connective tissue components.28-30 Previous reports have shown that bile duct ligation in the rat for four weeks produces cirrhosis and a sixfold increase in liver collagen content.31-33 Our results show that genistein is able to ameliorate the cholestasis, fibrosis, liver architecture, and biochemical markers of liver damage when administered to rats that had been bile duct ligated for four weeks. The mechanism of the action of this compound is probably associated with its ability to reduce the proliferation of HSCs and then the production of hepatic collagen, as well as by increasing matrix degradation; however, the possibility of other actions cannot be disregarded.
The major obstacle to antifibrotic drug development is the slow evolution of fibrosis, which takes years or even decades in humans. Consequently, there is an evident need for an effective treatment with the aim of modifying the clinical course of this disease. A recent insight into the molecular pathogenesis of hepatic fibrosis and the role of activated HSCs provides hope for future development of successful therapy. Genistein is a new drug with hepatic protective properties that may be beneficial in liver fibrosis.
Fibrosis is a well-known phenomenon that leads to loss of normal architecture and function. Thus, restoration of liver homeostasis (i.e., regulation of serum bilirubins and enzymes) by genistein could be explained, at least in part, by the antifibrotic effect of this compound. However, the amelioration of serum bilirubins and enzyme activities cannot be fully explained with the present data. One possibility is that genistein affected the serum bilirubin and enzyme concentration by mechanisms other than by preventing fibrosis. These mechanisms could include decreased production of bilirubins and enzymes or their increased elimination via an anti-oxidative pathway.
Natural flavonoids possess reactive phenolic groups and show antioxidative properties in vitro. Aneja et al.34 showed that the pretreatment of animals with genistein markedly increases the intracellular reduced glutathione (GSH) levels in animals treated with CCl4 and restores them to normal levels. They suggest that the induction of GSH levels may be due to the enhancement of GSH synthesizing enzymes such as c-glutamyl cysteine synthetase and GSH synthetase, which are key enzymes in its biosynthesis.35-36 They also speculate that genistein may cooperate with physiological defense molecules such as reduced glutathione in such a way as to protect animals against oxidative stress. Recently, Lee et al.14 reported that genistein at higher levels decreased hepatic fat accumulation possibly by increasing fatty acid oxidation and uncoupling protein; in low doses, genistein increased mitochondrial enzyme activities in mice with fatty liver and obesity induced by high-fat diets. Taking these data all together could explain why genistein ameliorates liver function in bile duct-ligated rats.
There is a wealth of evidence that HSCs orchestrate most of the important events in liver fibrogenesis. After liver injury, HSCs become activated to a profibrogenic myofibroblast phenotype and can regulate net deposition of collagens and other matrix proteins in the liver.8932 it has been shown that genistein is able to inhibit PDGF-driven proliferative activity of rat HSCs 2, and also inhibits the TGF-31-stimulated collagen synthesis.23 Genistein also influences proliferation of HSCs, suppresses the expression of a-SMA in HSCs, and inhibits the intensity of c-fos, c-jun, and cyclin D1 expression of HSCs.37 In this work we have shown that genistein reduced the total amount of liver collagen and ameliorated the liver architecture; therefore, it is possible that genistein also influences the HSCs in vivo.
The effects of genistein on other hepatic cells have also been studied. The inhibition of cell proliferation and the induction of apoptosis via activation of caspase-3 have been observed in genistein-treated liver cancer-bearing animals.38 It has been shown that genistein modulates gene expression in hepatocytes.19 On the other hand, it has been observed that 100, amol/L genistein increased the synthesis of nitric oxide by sinusoidal endot-helial cells from the early stage (stage I) of fibrosis.39 Those data suggest that genistein may play an important role in regulating the function of all cells residing in the liver, not only in physiological conditions, but also in liver disease.
Extracellular matrix deposition is a constant feature in liver cirrhosis. Proteolytic enzymes are thought to play a primary role in the degradation of the connective tissue generated in processes such as fibrosis.40 Different enzymatic systems may contribute to the overall process of ECM degradation, including plasminogen activators, matrix metalloproteinases (i.e., collagenases), and cathe-psin D, and it is likely that these enzymatic systems can act either independently or in a concerted manner.41 The involvement of these powerful proteolytic systems in pathologies such as hepatic fibrosis reinforces the idea that specific mechanisms must exist for its regulation and could be a target for therapeutic strategies in that process. Thus, the increase in the proteolytic activity mediated by genistein administration may be one of the antifibrotic mechanisms of this compound. It has been reported that genistein can modulate the secretion of urokinase type plasminogen activator and other metalloproteinases in human cancer cell lines.42-44 Nevertheless, further investigations concerningthemodulationof the proteolytic activity in the liver disease by genistein are needed.
Insummary, the present report showed that genistein inhibits the fibrosis and cholestasis induced by prolonged biliaryobstruction in the rat. Genistein has therapeutic potential against liver fibrosis.