Background and rationale. Portal hypertension (PHI) is a clinical syndrome characterized by increases of the blood flow and/or of the vascular resistance in the portal system. A direct consequence of PHI can appearance different lesions on the gastric mucosa and submucosa, cumulatively termed portal hypertensive gastropathy (PHG). Aims. To investigate the effects of glutamine on oxidative stress in an experimental model of PHG induced by partial portal vein ligation (PPVL).
Material and methods. Portal pressure, transaminase and alkaline phosphatase activity were quantified. Gastric tissue damage was assessed by histological analysis. Oxidative stress was measured by quantification of cytosolic concentration of thiobarbituric acid reactive substances (TBARS), hydroperoxide-initiated chemiluminescence (QL), and nitric oxide (NO) production. Moreover, activities of the antioxidant enzymes superoxide dismutase (SOD), glutathione pe-roxidase (GPx), and catalase (CAT) were analyzed.
Results. Transaminase and alkaline phosphatase activities were not significantly modified by PPVL, indicating absence of liver injury. Histological analysis of gastric sections showed a lost of normal architecture, with edema and vasodilatation. TBARS, QL, and NO production were significantly increased in PPVL animals. A reduction of SOD activity was found. Glutamine administration markedly alleviated histological abnormalities and oxidative stress, normalized SOD activity, and blocked NO overproduction.
Conclusions. Our results confirm that the use of molecules with antioxidant capacity can provide protection of the gastric tissue in portal hypertension. Glutamine treatment can be useful to reduce the oxidative damage induced by PHI on gastric tissue.
Portal hypertension (PHI) is a clinical syndrome which is usually secondary to intrahepatic or extra-hepatic obstruction of portal flow. Increased resistance to portal blood flow is the primary factor in the pathophysiology of portal hypertension.1 As the portal pressure elevates, portal-systemic collaterals develop gradually to diverse blood flow from the portal system. Gastroesophageal varices are most prominent collaterals and gastroesophageal variceal hemorrhage can lead to a high morbility and mortality. Different lesions characterized by marked dilatation of the gastric mucosa and submucosa, with a mosaic-like pattern with or without red spots, have been detected in both patients with cirrhotic or non-cirrhotic portal hypertension.2,3 These morphological alterations have been cumulatively termed portal hypertensive gastropathy (PHG), as they are considered a direct consequence of PHI.4
The lesions of the gastric mucosa observed during endoscopy in patients with portal hypertension and cirrhosis are very common, occurring in between 7 and 98% of cases, according to different series.4,5 Approximately 65-90% of those patients have mild PHG, whereas 10-25% of patients have severe PHG.6 The likelihood of developing PHG is thought to be dependent on the aetiology of portal hypertension and the severity of liver disease. However, PHG can occur in patients who do not have cirrho-sis,7,8 in patients awaiting liver transplantation,9 or in patients undergoing transjugular intrahepatic portosystemic shunt (TIPS) therapy for portal hypertension associated bleeding.10
The molecular mechanisms involved in the patho-genesis of PHG have not been fully elucidated. Cellular redox state is a consequence of the precise balance between the levels of oxidizing and reducing equivalents, such as reactive oxygen species (ROS) and endogenous antioxidants. ROS are kept at physiologically optimal levels under normal conditions by antioxidant defense systems, including the enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). Perturbation of this equilibrium due to increased ROS production and/or reduced antioxi-dants leads to oxidative stress. A role for oxidative stress in the development of the hyperdynamic circulation associated to PHI has been proposed by some au-thors,11 and recently gastric tissue oxidative changes in patients with PHG have been described.12
Increases in the synthesis of nitric oxide (NO) have also been reported in liver of rats with PHI.13 Moreover, NO production has been implicated in the pa-thogenesis of PHG, with increases in NO serum levels in patients with PHG. The enhanced synthesis of NO induces hyperdynamic circulation and peroxynitrite overproduction by reaction with ROS, increasing the susceptibility of gastric mucosa to damage.3,14
Glutamine, a nonessential amino acid, has received increasing attention because it becomes essential during stress and catabolic conditions.15,16 Glutamine administration can result in an enhanced antioxidant capacity in different situations such as critical illness or sepsis.17 In stomach, glutamine is able to protect against peptic ulceration, and improves the healing process of ulcers.18 Because glutami-ne plays a role in the efficient treatment of various diseases, the present study was designed to investigate the potential beneficial effects of glutamine administration on gastric oxidative stress, NO production, and the histological modifications in an experimental model of PHG.
Material And MethodsAnimals and experimental groupsMale Wistar rats with mean weight of 250 g were used. Animals were obtained from the Center for Breeding of Laboratory Animals (CREAL) of the Federal University of Rio Grande do Sul (UFRGS). Rats were caged at 20-24 °C, with a 12 h light/dark cycle, and free access to food and water. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). The rats were randomly divided into four groups of fourteen animals each:
- •
S (sham- operated rats receiving only ClNa as vehicle).
- •
S+G (sham-operated rats receiving glutamine).
- •
PPVL (PPVL rats receiving vehicle).
- •
PPVL+G (PPVL rats receiving glutamine).
During the procedure, rats were anesthetized with ketamine chlorhidrate (Ketalar, Parke Davis, 100 mg/kg), and xilazine 2% (Rompun, Bayer, 50 mg/kg) cocktail i.p. PHI was induced by partial portal vein ligation (PPVL) as described by Moreira, et al.4 Briefly, the portal vein was isolated and a 3-0 silk ligature was tied around both the portal vein an adjacent 20 gauge blunt-tipped needle. The needle was then removed, and the vein allowed to re-expand. A second loose ligature was left around the portal vein with two endings of the ligature placed on each side in the abdominal cavity. The abdomen was then closed and the animal allowed to recover. Control rats underwent a similar operation but without occlusion of portal vein. Sham operated animals received only vehicle (ClNa, 1 mL/kg, i.p.). Glutamine was administrated daily (25 mg/kg, daily, i.p.) by 7 days, beginning on the eighth day after the surgical protocol. All rats were anesthetized and sacrificed on the fifteenth day of the protocol. Stomachs were immediately removed and blood samples were centrifuged at 1,800 g for 15 min at 4 °C to obtain plasma.
HistologyFor histological examination a piece of the stomach was trimmed, and fixed by immersion in Bouin’s solution for 12 h, and then transferred to 10% buffered formalin. The blocks were dehydrated in a graded series of ethanol and embedded in paraffin wax. Serial 3-μm. It was performed a semi-quantitative analysis, being used as a parameter 0 to 4 (absent, very small amount, small, moderate and heavy), by two observers.
Sections were stained with hematoxilin and eosin. Five sections from each sample were analyzed by two independent pathologists who had no prior knowledge of the animal groups.
Hemodynamic studies and plasma enzyme activitiesPortal pressure (PP) was evaluated by cannula-ting the superior mesenteric vein with PE-50 catheter connected to a pressure transducer (Braun AG, Mel-sungen, Germany). PP recordings were made on a Letica polygraph (Letica, Rochester, MI, USA). The plasma activity of aspartate animotransferase (AST), alanine aminotransferase (ALT), and alkaline phos-phatase (ALP) was estimated by commercially available kits (Boehringer, Mannheim, Mannheim, Germany).
Oxidative stress determinationsGastric oxidative stress was evaluated by measuring both the concentration of aldehydic products (TBARS), and the hydroperoxide-initiated chemilu-minescence (QL). Briefly, the frozen tissue was homogenized in 140 mM KCl, 20 mM phosphate buffer (pH 7.4) and centrifuged at 14,000 g for 10min. For TBARS analysis the amount of alde-hydic products generated by lipid peroxidation was measured by the thiobarbituric acid reaction using 3 mg of protein per sample. The samples were incubated at 90 °C for 30 min after adding 500 mL of 0.37% thiobarbituric acid in 15% trichloroacetic acid, and then centrifuged at 2,000 g for 15 min. Spectrophotometric absorbance was determined in the supernatant at 535 nm.19 For QL determination, 0.5 mL of homogenate were added to 120 mM KCl, 30 mM phosphate buffer (pH 7.4), and 3 mM tert-butyl hydroperoxide at 30 °C and assayed for chemiluminescence in a liquid scintillation counter in the out-of-coincidence mode.
NO quantificationNitric oxide production in the gastric tissue was measured indirectly using a quantitative colorime-tric assay based on the Griess reaction. This method is sensitive for both nitrite and nitrate ions.20 Briefly, the samples were deproteinized and subsequently centrifuged for 20 min at 12,000 g. After incubation of the supernatants with E.coli nitrate reductase (37 °C, 30 min) to convert nitrates to nitrites, 1 mL of Griess reagent (0.5% naphthylethyle-nediamine dihydrochloride, 5% sulfonylamide, 25% phosphoric acid) was added. The reaction was performed at room temperature for 20 minutes, and ab-sorbance at 546 nm was measured, using a sodium nitrate solution as standard.
Antioxidant enzyme activitiesCytosolic superoxide dismutase (SOD; EC 1.15.1.1) was assayed spectrophotometrically by rate of epine-phrine auto-oxidation, which is progressively inhibited by amounts of SOD in the homogenate, the amount of enzyme that inhibits auto- oxidation at 50% of the maximum inhibition is defined as 1 unit of SOD activity.21 Glutathione peroxidase (GPx, EC 1.11.1.19) was carried out according to Flohé and Gunzler,22 cummene hydroperoxide was used as the substrate, and 1 unit of enzyme activity was defined as the amount of protein that oxidizes 1 μmol of reduced NADPH/min. Catalase (CAT; EC 1.11.1.6) activity was determined by measuring the exponential disappearance of H2O2 at 240 nm.23
Statistical analysisResults are expressed as mean values ± SEM. The data were compared by analysis of variance (ANOVA); when the analysis indicated the presence of a significant difference post hoc comparisons were carried out using the Newman-Keuls test. Statistical significance was set at p < 0.05. Data were analyzed using the statistical software SPPS+ version 15.0 (SPSS, Inc., Chicago, IL).
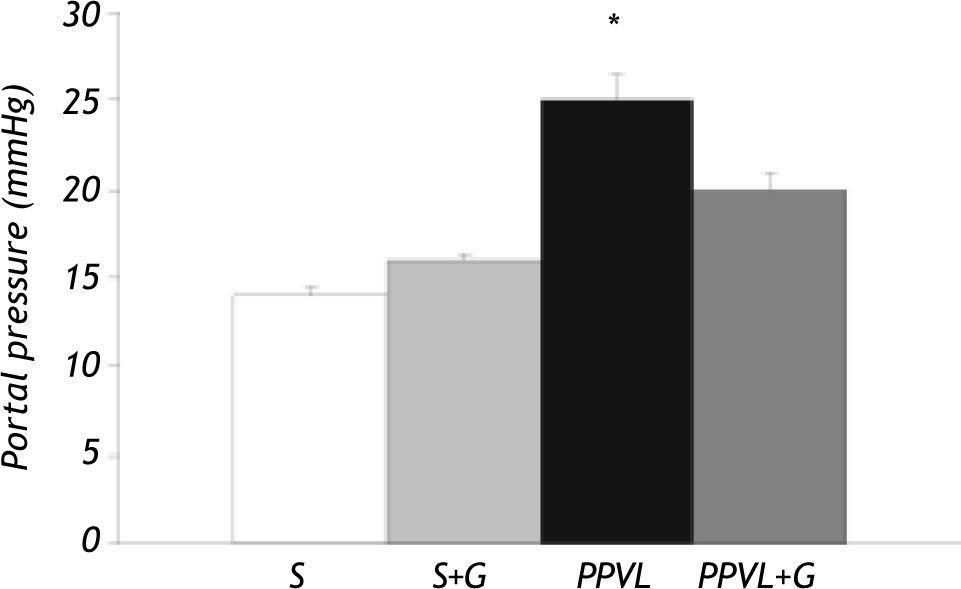
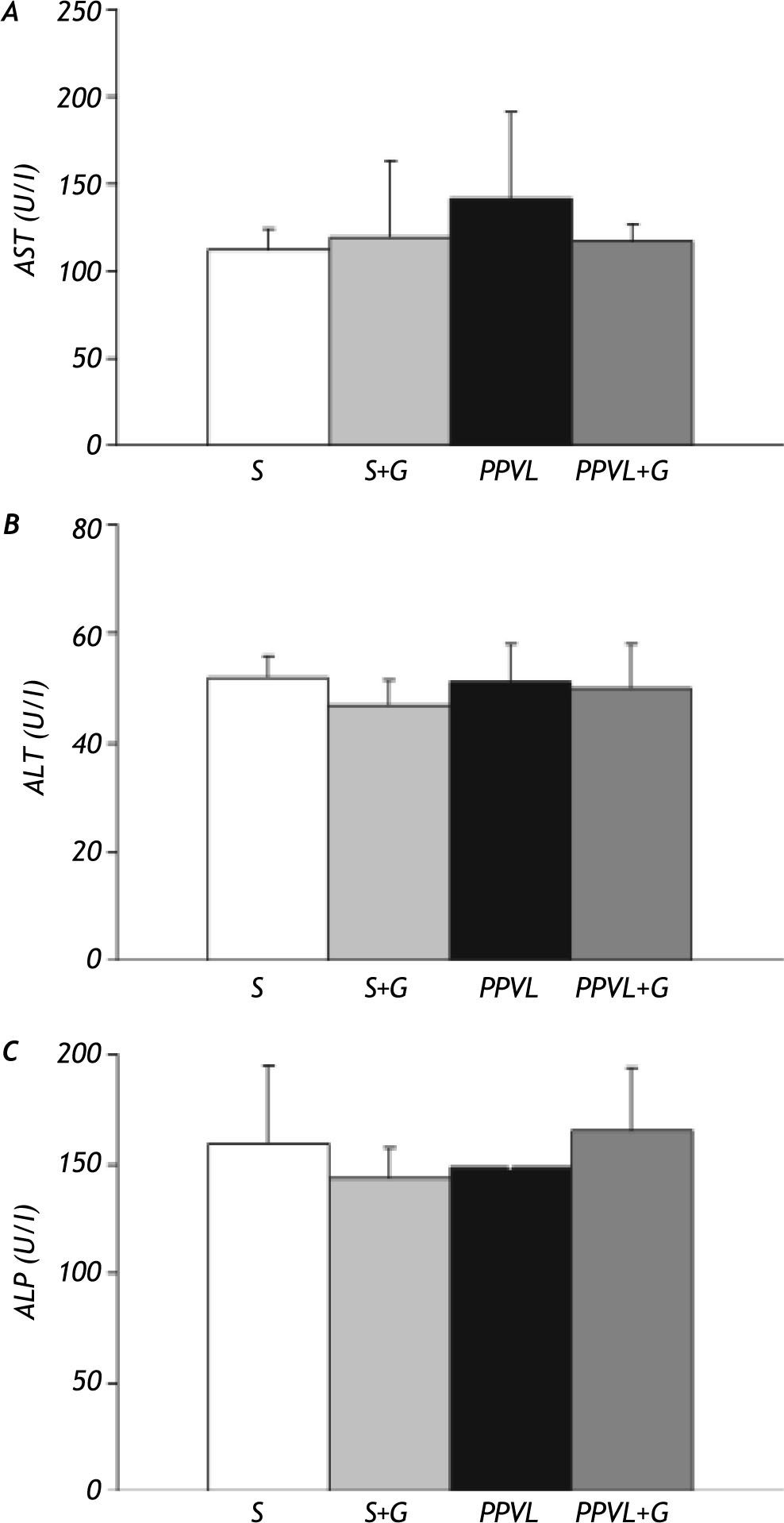
ResultsPortal pressure and transaminase activities. There was a significant difference in portal pressure between sham-operated rats and those with portal vein ligation (+80%). This increase was partially prevented by glutamine administration in PPVL+G rats (Figure 1). Transaminase and alkaline phosphatase activities did not significantly differ between the different experimental groups (Figure 2).
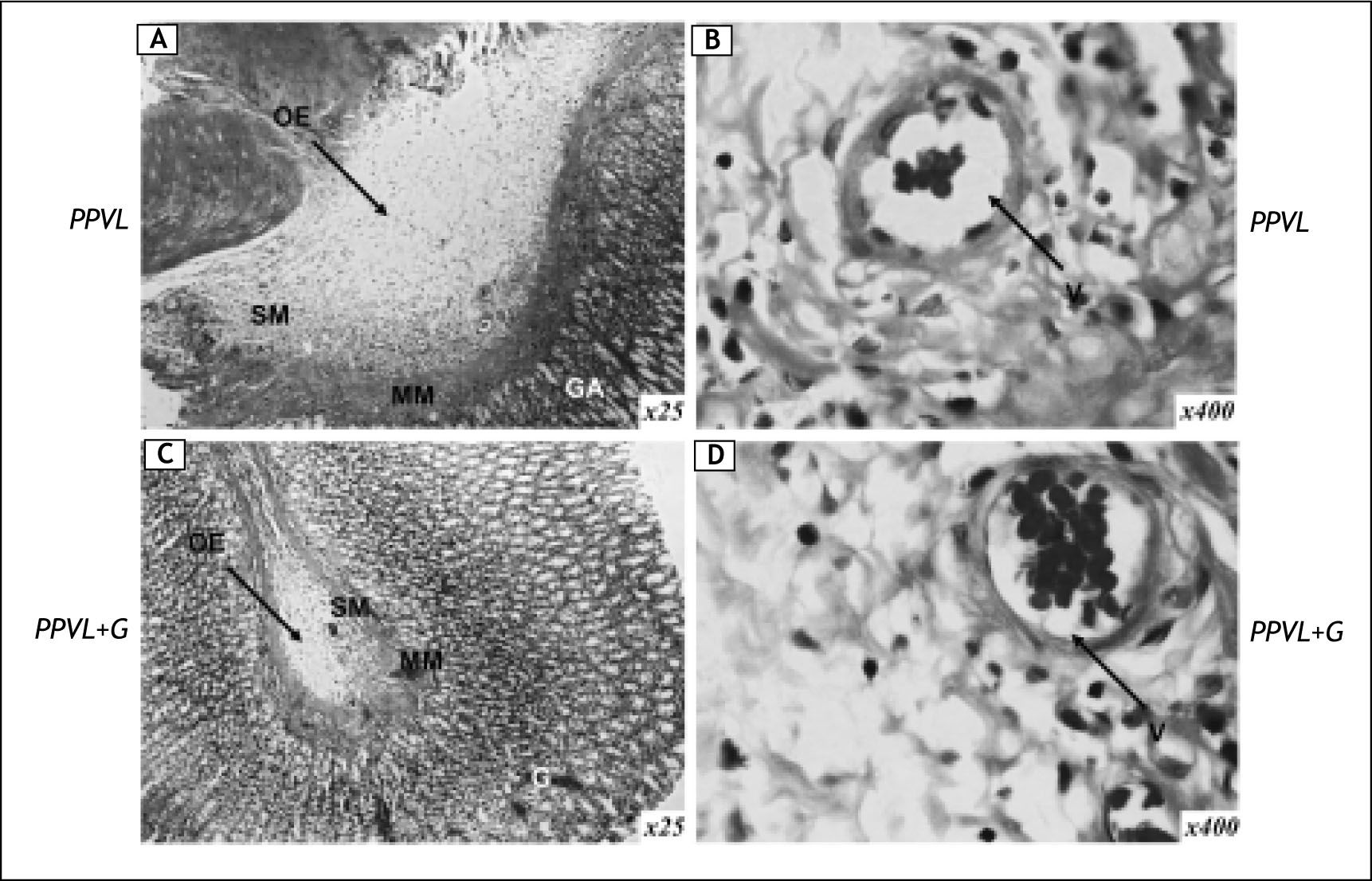
Histological examination of gastric sections in PPVL animals showed modifications of normal structure, with congestion and oedema in the sub-mucosa, vasodilatation and proliferation of blood vessels (Figure 3A and 3B). Administration of gluta-mine markedly alleviated the histological abnormalities (Figure 3C and 3D). No histological changes were detected in the livers of different groups (data not shown).
Representative micrographs of gastric tissue in rats with partial portal vein ligation (PPVL) (A and B) and rats with portal vein ligation and glutamine treatment (PPVL + G) (C and D). G: glandular tissue. MM: muscular mucosa. SM: submucosa. Hematoxilin and eosin staining; original magnification x 25 (A and B), x 400 (B and D). Oedema (OE) and vasodilatation (V) were evident in PPVL-rats. Glutamine treatment was able to ameliorate histological changes.
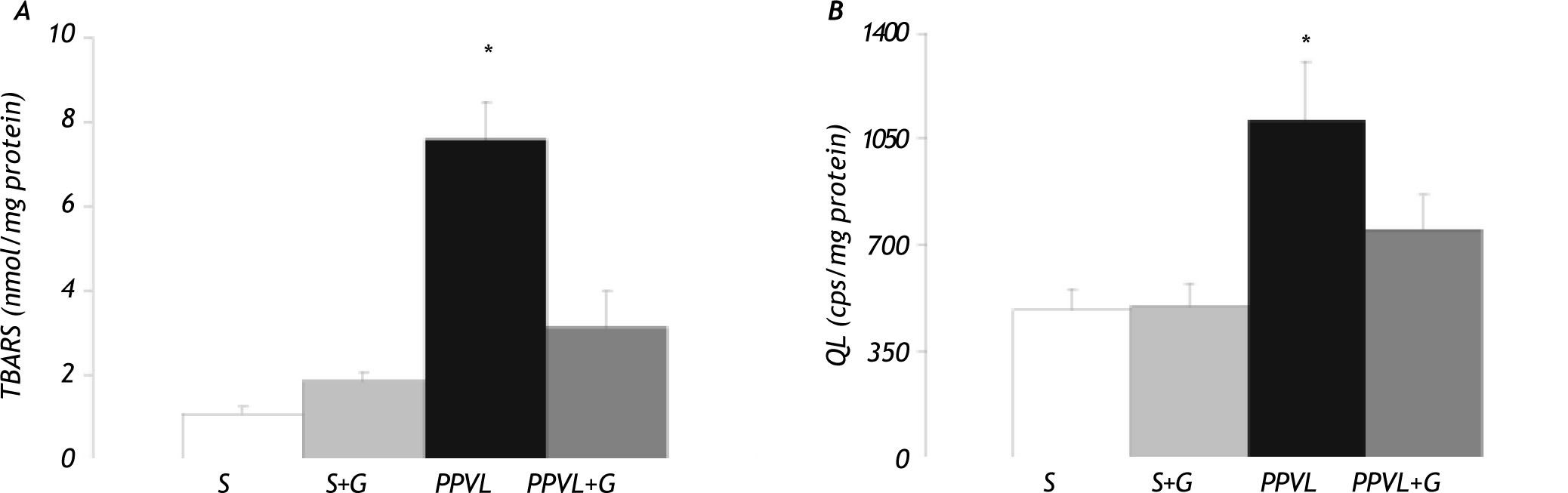
The cytosolic concentration of TBARS increased in the animals with portal hypertension (PPVL group) (+ 620%), while values did not significantly differ from the controls in PPVL rats treated with glutamine (Figure 4A). A significant increase in li-poperoxidation measurement by hydroperoxide-ini-tiated chemiluminescence (QL) was also observed in PPVL rats (+ 127%). This increase was precluded by the administration of glutamine in PPVL+G rats (Figure 4B).
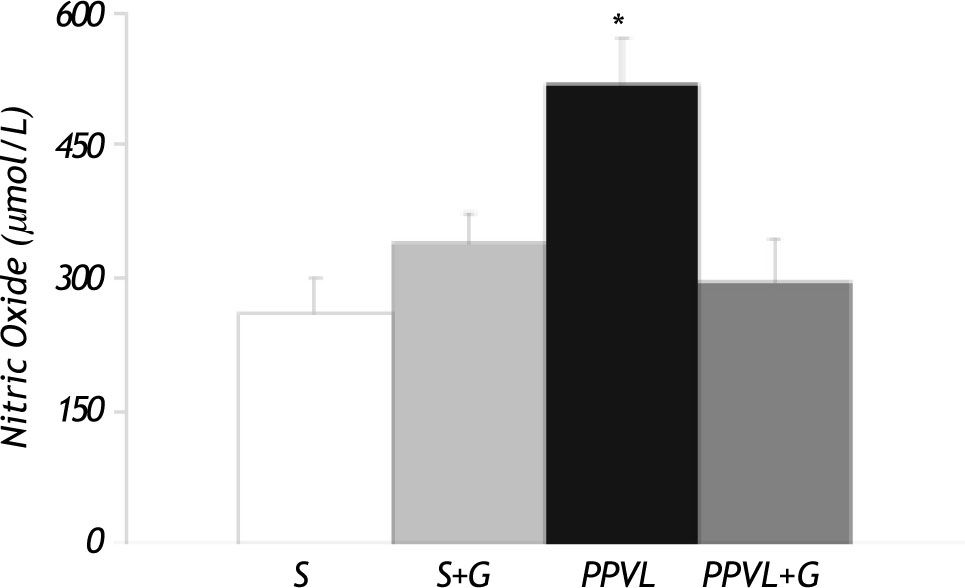
Nitric oxide levelsFigure 5 shows concentration of nitrites in gastric tissue. Values were significantly higher in PPVL rats (+ 100%). Moreover, this parameter was completely normalized in animals receiving glutamine.
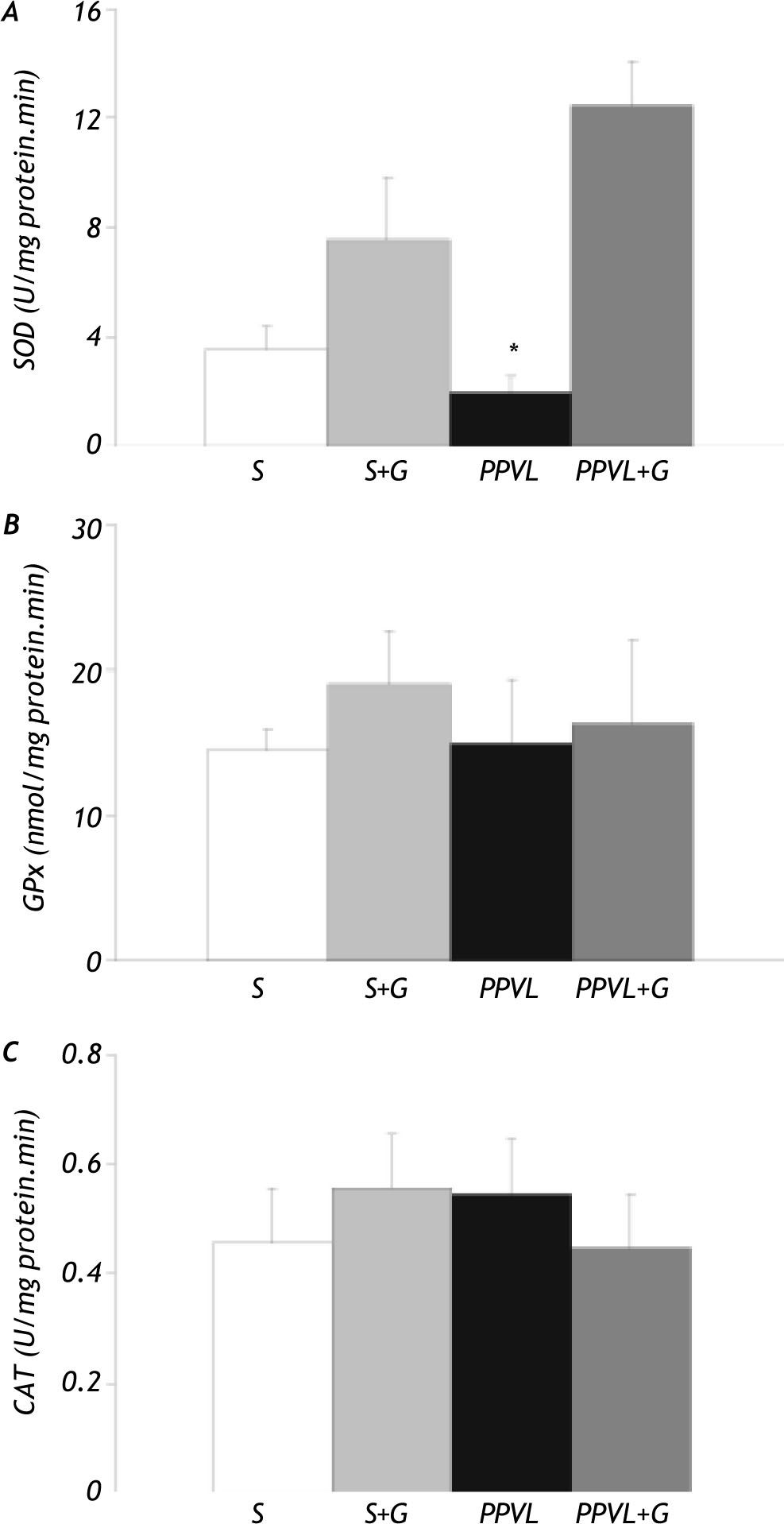
Antioxidant enzyme activitiesAnalysis of antioxidant enzyme activities showed that portal vein ligation induced an important reduction of gastric SOD activity in the PPVL group (-45%). Values were increased by glutamine administration. However, no significant difference in both GPx and CAT activities was found between the different experimental groups (Figure 6).
Effect of partial portal vein ligation (PPVL) and glutamine (G) administration on gastric antioxidant enzyme activities. A. Superoxide dismutase (SOD) activity. B. Glutathione peroxidase (GPx) activity. C. Catalase (CAT) activity. Values are means ± SEM for 14 rats. *p < 0.05, against sham-operated group.
PHG is recognized as a clinical entity in PHI, but the exact pathogenesis of PHG is still unclear. The PPVL animal model has been extensively studied and found to be a useful tool for understanding the pathophysiology of PHI and PHG.3,24 This model has been developed in different animal species such as rats, mice or rabbits,25 and it is presently accepted to be suitable for investigating the pathogenesis of PHI and PHG, because is very reproducible and easy to perform, and portal hypertension develops rapi-dly.25 The model used in our study is characterized by prehepatic portal hypertension, with a maintained hepatic structure, an hyperdynamic circulation and portal-systemic shunting.24,25 The percentage of portal-systemic shunting approaches 100% after the 7th. day. Moreover, mesenteric vasodilatation and increased cardiac output are detectable at the 4th day.24 This model can provide measurements in the portal circulation which cannot be performed accurately in humans because of ethical and technical limitations.
In our experimental work, portal hypertension was accompanied by the presence of edema and dilated vessels in the gastric submucosae (Figure 3) but no alteration was seen in liver histology or transa-minase activities (Figure 2). This confirms previous findings that the procedure of PPVL, although causing a transient reduction in the metabolic activity of the liver,26 does not produce hepatocellular dama-ge,27 and supports the suggestion that portal hypertension seems to be the key factor for the development of PHG, being equally common in portal hypertensive patients with or without liver di-sease.28 Different authors have demonstrated that PPVL rats show abnormalities of the gastric micro-vasculature comparable to those observed in PHG in humans.25,29 In our study glutamine administration was able to reduce portal hypertension and ameliorate all the gastric histopathological changes, with reduction of edema and vasodilatation. It has been described that administration of a mixture of aminoacids containing glutamine is able to reduce the histological changes on acid-induced gastric damage in starved rats.30 Moreover, other studies show that glutamine is able to protect against peptic ulceration, and improves the healing of ulcers in stomach.31
Oxidative stress can be the result of excessive generation of reactive oxygen species (ROS), depletion of intracellular defenses or a combination of both, leading to an imbalance in the redox status of cell. ROS overproduction have been implicated on several pathophysiological situations,20,32 and have been also described in PHI and PHG.3,33 Moreover, the fact that PPVL results in oxidant injury was first demonstrated by Fernando, et al.,34 which concluded that formation of ROS may be important in the pathogenesis of hemodynamic changes and the development of the hyperdynamic circulation. Our data demonstrate that glutamine is able to induce a significant reduction of oxidative stress induced by PHG, as indicated by the lowering of TBARS concentration and hydroperoxide-initiated chemolumi-nescence (Figure 4). Different studies have shown the antioxidant capacity of different aminoacids or their derivates, such as cysteine, methionine, glycine, glutamine, etc.3 Glutamine is required for gluta-thione synthesis, the most abundant intracellular thiol and antioxidant. A decrease of glutathione levels in portal hypertensive rats has been reported by different authors. Studies about the effect of toxic agents on the gastric mucosa have revealed that a decrease in glutathione (20-25%) can lead to significant damage to the gastric mucosa by lipid peroxi-dation.31 Moreover, it has been described that enteral glutamine supplementation in an animal model of ileitis enhances the intestinal glutathione con-tent,35 and it has been reported that prophylactic glutamine administration is associated with decreased TBARS and increased glutathione levels in colonic mucosa of rats with TNBS-induced colitis.36 Studies have demonstrated increased oxygen free radicals and lipid peroxidation in the pathogenesis of HP in gastric mucosa. Demonstrating the involve-ment of lipid peroxidation, but also an increase in the levels of nitrotyrosine in gastric mucosa.37 Some research, conducted with other antioxidants like vitamin E and lazaroid show the reduction of lipid pe-roxidation in a model of intestinal injury scheme and HP, respectively, induced by oxidative stress. These studies demonstrated a reduction of lipid pe-roxidation in experimental groups subjected to these models.38 Therefore, antioxidant properties of gluta-mine39 could contribute to their beneficial effects in experimental PHG.
Nitric oxide (NO), is a potent vasodilatador, is generated from L-arginine by an enzyme called NO synthase (NOS). Distinct cDNAs for NOS enzyme have been described for an inducible isoform (iNOS) and 2 constitutive isoforms (eNOS and nNOS).40 Studies have shown the activation of eNOS and, although this increase in their levels may explain the increased levels of NO in gastric mucosa of HP animals, we know the existence of other pathways involved in this mechanism. For example, via PI 3-kinase-Akt. These data suggest that activation of eNOS in gastric mucosa is not only caused by the increase of eNOS protein, but is also caused by direct phosphorylation of eNOS through the activation of PI 3-kinase-Akt and contributes to increase NO production.41 Molecular studies indicate that the lesion secondary to HP in the gastric mucosa induced by alcohol may inhibit ERK2 activation in response to injury, with an elevation of MKP-1. Research conducted in transfected cell lines shows an overexpression of MPK-1, suggesting an inhibition of ERK, indicating a pre-expression of MPK-1 in lesions of HP, in an experimental model induced by alcohol.42
In the gastric mucosa, NO can play different roles. NO protects the gastric mucosa against injury by ethanol and endothelin-1, whereas the inhibition of NO can increase the gastric mucosal injury.43 However, the excessive increase of NO production has a cytotoxic potential, enhancing mucosal injury. We have detected an increased production of NO in our experimental model, which can be related to the induction on eNOS, nNOS, and iNOS mRNA expression, previously described in portal hypertensive gastric mucosa.44,45 Excessive NO synthesis, associated with ROS production, plays an important role in the increased susceptibility to gastric damage by peroxynitrite overproduction, which initiates membrane lipid peroxidation and thus contribute to cell injury.46 As a result, NO and peroxynitrite overproduction may be an underlying mechanism for the increased susceptibility to damage and is probably related to the pathogenesis of PHG. Some authors have described a reduction on gastric damage by inhibition of endogenous NO production, which may be in part associated with a significant decrease in expression of gastric mucosa iNOS mRNA.35 A previous report of our group has shown that the administration of quercetin, an antioxidant molecule, is able to reduce NO production by a reduction of iNOS protein level in PPVL rats.3 Considering that NO levels rise in the experimental groups, this could be the explanation for the reduction of portal pressure by glutamine. And NO produced through the action of NOS on L-arginine, which are released from L-citrulline and NO. Reliant addition, it is suggested that L-arginine would have a limited role in the presence of glutamine, inhibiting the formation of NO by reducing portal pressure.46,47 Furthermore, in a rat model of acetic acid-induced colitis, we have also found that glutamine administration can reduce both iNOS expression and oxidative stress.48
Antioxidant enzymes such as SOD, GPx and CAT play critical roles in oxidative stress protection by converting ROS into less harmful products. Our results indicate that LLPV induces an important reduction of the SOD activity, without changes on GPx and CAT activities. The decrease of SOD activity is in line with previous studies which have shown a similar effect in NSAID-induced gastropathy and in PPLV rats.3,48 The drop of SOD activity could be explained by the increase in NO production. In this way, it has been previously described that SOD inac-tivation can be induced by NO overproduction; moreover, administration of NO synthase inhibitors can prevent the loss of SOD activity.4 This reduction may enhance lipid peroxidation as well as aggravate the injury to gastric mucosa. In our study, glutamine administration was able to block the reduction on SOD activity, thus contributing to normalize the gastric antioxidant defense. Although the potential role of antioxidant therapy has not been studied widely, our group has described previously that the administration of quercetin was able to normalize SOD activity and reduce oxidative stress, alleviating gastric damage induced by PHI, in PPLV rats.3 The non-significant increase in SOD activity induced by glutamine even in absence of PHI is difficult to explain, but could be, at least partly related to the induction of superoxide generation that has been occasionally reported in different cell types. Moreover, the increase in SOD activity in PPLV group can be related to its capacity to block the NO overproduction by reduction of iNOS expression in portal hypertensive gastric mucosa,49 ameliorating the SOD inactivation by NO.50
In this paper, we describe the beneficial effects of glutamine treatment on oxidative stress, but more experimental studies are needed to better understand the molecular mechanisms of action of this amino acid in hypertensive gastropathy. The reduction of portal pressure, associated with a marked reduction of histological changes, the normalization of SOD, the reduction in NO production and inhibition of lipid peroxidation induced by glutamine, confirms that use of molecules with antioxidant capacity could provide a new therapeutic modality for protecting the gastric tissue in portal hypertension. Studies have shown that the formation of L-citrulline from L-arginine is inhibited by high levels of gluta-mine. As the formation of NO occurs through the action of NOS on L-arginine, it is suggested that glutamine acts by inhibiting the release of endothe-lium-derived NO, the main modulator of HP, thus reducing the pressure in the splanchnic.
This work was supported by grants from the Conselho Nacional de Desenvolvimiento Científico e Tecnológico (CNPq), Fundaçáo de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS), and Fundo de Incentivo á Pesquisa e Eventos (FIPE) do Hospital de Clínicas de Porto Alegre (HCPA). CIBEREHD is funded by the Instituto de Salud Carlos III.