Hepatic encephalopathy (HE) is a complication that presents in as many as 28% of patients with cirrhosis, and reported up to ten years after the diagnosis of cirrhosis. Commonly, it is observed in patients with severe hepatic failure and is characterized by neuropsychiatric manifestations that can range in severity from a mild alteration in mental state to a coma; additionally, some neuromuscular symptoms can be observed. This complication of either acute or chronic hepatic disease is the result of a diminished hepatic reservoir and inability to detoxify some toxins that originate in the bowel. Today, the role of astrocytes, specifically the Alzheimer type II cells, is known to be very important in the pathogenesis of the hepatic encephalopathy, and will be reviewed later.
In conclusion, the objectives of this review are:
• To understand the pathogenesis of hepatic encephalopathy
• To recognize the precipitating factors, as well as preventive measures for the development of the hepatic encephalopathy
• To describe the new classification of hepatic encephalopathy and its clinical implications
• To recognize the clinical manifestations and stages of the disease
• To understand the main diagnostic tests used to detect the hepatic encephalopathy
• To describe the main therapeutic treatments of hepatic encephalopathy
Hepatic encephalopathy is a potentially reversible, or progressive, neuropsychiatric syndrome characterized by changes in cognitive function, behavior, and personality, as well as by transient neurological symptoms and characteristic electroencephalographic patterns associated with acute and chronic liver failure.
Hepatic encephalopathy is a frequent complication of cirrhosis that is usually observed in association with severe hepatic insufficiency.1 The characteristic presentation is the development of acute encephalopathy with an abrupt decline in the level of consciousness, manifested as confusion or coma. Frequently, a precipitating factor can be identified. The treatment of the episode is directed toward the correction of the precipitating factor. Once the precipitating condition is resolved the encephalopathy also typically disappears, with the patient recovering to his or her previous state. However, in patients with low reserves of hepatic function, the hepatic encephalopathy can be a chronic condition. The low reserve predisposes the patient to development of spontaneous hepatic encephalopathy. There is usually a precipitating factor, and the diagnosis and treatment should consider such aspects.
Chronic encephalopathy can manifest as frequent episodes of acute encephalopathy (chronic-recurrent encephalopathy) or with persistent neurological manifestations (chronic-persistent encephalopathy). The distinction between the two forms is subjective and is reflected in the prominence of neurological manifestations. In practice, the distinction can be difficult because acute episodes coexist with the chronic manifestations; if the manifestations are mild, the term “recurrent encephalopathy” should be used. On the other hand, if the chronic manifestations are severe, the term “persistent encephalopathy” is appropriate.
In patients with chronic-recurrent encephalopathy, episodes can be associated with a precipitant factor but generally are spontaneous or related to the termination of treatment. It is not infrequent that these episodes are attributed to constipation, associated with administration of an inappropriate dose of non-absorbable disaccharide. An acute episode of spontaneous encephalopathy usually has an abrupt onset and termination. Between episodes, the patient can be alert and not display signs of cognitive dysfunction, unless he or she is examined by neuropsychological testing.2 Not infrequently, the patients present with a mild Parkinsonism, characterized by bradykinesia without tremor.
Since hepatic transplantation is a viable option, and since to a lesser extent patients can also be treated with surgical portal-systemic shunts, chronic-persistent encephalopathy is less frequent than the recurrent form. The characteristic manifestations of recurrent encephalopathy are the development of dementia, severe Parkinsonism or myelopathy that can be associated with other neurological manifestations (ataxia, dysarthria, and tremor).2 In these patients, the cognitive alterations have a sub-cortical pattern: attention deficits, alterations in vision and practice, and the apraxia predominate. Most of the time these effects are transient, while bradykinesia and symmetric rigidity predominate. Hepatic myelopathy presents as a spastic paraparesia with signs of a pyramidal involvement, but the senses are rarely affected.3
PathogenesisFor many years, controversy has existed about the origin of the toxins responsible for the altered mental state. There has been debate about the role of ammonium, synergic toxins, GABA or endogenous benzodiazepines in the development of hepatic encephalopathy. Today, it has been established that there are peripheral multi-organic alterations, as well as alterations in the intercellular communication of the brain, produced by alterations in astrocytes.
Peripheral alterations- a.
Intestinal
There is controversy about the role of Helicobacter pylori, which produces ammonium in the stomach, in the pathogenesis of hepatic encephalopathy. Some trials have demonstrated a high prevalence of the infection in individuals with alcoholic hepatitis who develop hepatic encephalopathy, as well as individuals with cirrhosis and chronic encephalopathy. However, eradication of H. pylori does not interfere with the levels of ammonium in this group of patients, and its contribution to the development of hepatic encephalopathy may therefore be minimal.
- b.
Portal-systemic communication
It has been demonstrated that some congenital abnormalities that cause portal-systemic shunts in children can be manifested by episodic hepatic encephalopathy, even without preexisting hepatic disease. Also, the cirrhotic patients with portal-systemic shunts develop encephalopathy easily compared with patients without portal-systemic shunts.
- c.
Hepatic failure
There have been many trials reporting that hepatic failure is the main cause of the development of hepatic encephalopathy, resulting from decreased hepatic functional capacity that diminishes the detoxification of ammonium, raising the plasma levels of this element, thereby producing the clinical manifestations of these patients.
- d.
Muscle
A reduction in the muscle mass of cirrhotic patients can predispose them to the development of hepatic encephalopathy. Muscle waste is explained not only by the presence of hepatic disease and the patient’s nutritional status, but also by elevation of some cytokines like tumor necrosis factor-alpha (TNF-α), which in turn activates transcription factors like NK-a, resulting in reduced myosin synthesis. Muscle waste is associated with a lower metabolic capacity to detoxify ammonium and glutamine, resulting in the development of hepatic encephalopathy.
- a.
Osmotic
Some studies have demonstrated that there are osmotic changes in patients with cerebral edema and hepatic insufficiency. On one hand the brain develops edema, raising intracerebral pressure and producing herniation that can result in death. The glutamine produced because of the detoxification of ammonium in the astrocytes is considered an organic osmol that can cause edema in the astrocytes. It has been observed that the water channel aquaphorin-4 drives water into the cell. There is also evidence that the brain adapts to changes during chronic hepatic disease. Direct and indirect determinations of the organic osmoles using spectroscopy by resonance imaging in humans have demonstrated a loss of myo-inositol, taurine and glyceryl-phosphocholine, which are the osmoles used by the astrocytes for the regulation the intracellular osmolality. These changes make the brain more vulnerable to a second osmotic change.
- b.
Axonal communications
There is also evidence of the important role of astrocytes in maintaining normal neuronal function. In hepatic encephalopathy there are no morphologic alterations in the neurons. The Alzheimer type II cells (astrocytes) can show some abnormalities: reduced activity of transporters (glutamate), increased expression of benzodiazepine receptors and increased monoamine oxidase (MAO) activity. As a result there are alterations in the metabolic communication between astrocytes and other cells. For example, astrocytes synthesize neurosteroids which activate GABA receptors and endogenous benzodiazepines receptors.
- c.
Endothelial communication with astrocytes: brain blood flow and hepatic encephalopathy.
Patients with hepatic encephalopathy have fluctuations in cerebral perfusion. Some experimental models have demonstrated an increase in cerebral perfusion in the presence of high levels of ammonium. This can be activated by intra-cerebral signals generated after the synthesis of glutamine in the astrocytes. Hypothermia and brain edema can also have an important role in the low cerebral perfusion demonstrated in experimental models.
Cirrhotic patients have decreased brain blood flow, probably secondary to peripheral vasodilatation. These alterations can cause a decrease in metabolic activity.
- d.
Other hypotheses
There are other hypotheses related to the pathogenesis of hepatic encephalopathy:
- •
Ammonium
Ammonium is fundamental to the pathogenesis of hepatic encephalopathy. After detoxification of ammonium by the astrocytes some neuro-chemical alterations occur. There are many factors that interact with ammonium, causing alterations in the astrocytes (hyponatremia, cytokine elevations, alterations in the ligands of astrocytes), thereby producing an anatomic substrate and neuro-chemical synergism that can increase the development of hepatic encephalopathy. However, ammonium levels do not correlate with the severity of encephalopathy.
- •
Endogenous benzodiazepines
The role of these substances in the alteration of GABA-ergic neurotransmission is not well understood. Some studies with flumazenil have not shown significant results; also, ammonium can activate GABA-ergic pathways by the synthesis of neurosteroids in astrocytes, as stated before.
- •
False neurotransmitters
A decrease in branched-chain amino acids can favor the entrance into the brain of aromatic amino acids, which are precursors of false neurotransmitters that alter glutamine synthesis. The clinical experience with ramified amino acids is of great interest because it is possible that the amino acids have a direct effect in muscle, increasing ammonium detoxification. Other neurotransmission pathways involved in the development of hepatic encephalopathy are serotonin (5-HT), opiates and catecholamines.
Other additional factors that can favor the development of recurrent hepatic encephalopathy episodes are nutritional status, especially in alcoholic patients who can have deficiencies in vitamins and micronutrients. One such example is a deficit in zinc, which is a cofactor in the urea cycle. Zinc supplementation, using a dose of 600 mg/day, has been studied in encephalopathy but has not demonstrated additional benefits.4 However, it seems reasonable to measure the plasma zinc levels and add zinc supplementation when these are low. Another issue that has recently been studied is gastric colonization by H. pylori, a microorganism that produces urease. Eradication of H. pylori can be beneficial in other diseases, but it is not associated with a lower ammonium level or an improvement in hepatic encephalopathy.5
To conclude, hepatic encephalopathy is the result of a combination of hepato-cellular insufficiency (liver failure), toxin accumulation and the establishment of a portal-systemic shunt. The main precipitating factor (without being an specific cause) is an altered level of plasma ammonium. The pathogenic mechanism includes the production of false neurotransmitters, facilitated sensibility of neurons by γ-aminobutyric acid (GABA), increases in the plasma endogenous benzodiazepines, diminished activity of urea cycle enzymes secondary to zinc deficiency and finally, manganese deposits on the basal ganglia.
Clinical stageGenerally, the diagnosis of hepatic encephalopathy is not difficult; the neurological exam is the main element to establish the diagnosis. As shown in table I, although stage 2 shows symptoms such as lethargy, significant confusion and behavioral changes, in stage 1 the behavioral changes can be minimal, such as euphoria, confusion or some degree of depression. The more advanced stages can be manifested as confusion, somnolence and coma. Also, some electroencephalographic changes can be found that vary between the presence of triphasic waves and delta activity.
Classification and grades of hepatic encephalopathy.
| Grade I. | Euphoria or depression, mild confusion, monotonous voice and/or sleep cycle disorders. Asterixis ± |
| Grade II. | Lethargy and/or confusion. Asterixis, triphasic waves on EEG |
| Grade III. | Severe confusion, incoherent language, semi-stupor but awakes with language. Asterixis, triphasic waves on EEG |
| Grade IV. | Coma, initially can respond to painful stimuli. Asterixis. Delta activity on EEG |
Recently, an expert group on hepatic encephalopathy described a new classification for patients according to the type of hepatic alteration that causes the condition,29,30 with three different types of encephalopathy being considered:
Type A: acute liver failure
Type B: portal-systemic bypass without intrinsic hepato-cellular disease (the more frequent)
Type C: cirrhosis and portal hypertension with portalsystemic shunts.
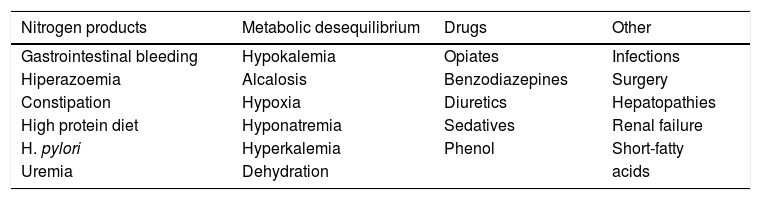
In a patient with previously stable cirrhosis, hepatic encephalopathy is usually a consequence of an easily identified precipitating factor, as shown in table II, with gastrointestinal bleeding the more common etiologic factor.
Predisposing factors for development of hepatic encephalopathy.
| Nitrogen products | Metabolic desequilibrium | Drugs | Other |
|---|---|---|---|
| Gastrointestinal bleeding | Hypokalemia | Opiates | Infections |
| Hiperazoemia | Alcalosis | Benzodiazepines | Surgery |
| Constipation | Hypoxia | Diuretics | Hepatopathies |
| High protein diet | Hyponatremia | Sedatives | Renal failure |
| H. pylori | Hyperkalemia | Phenol | Short-fatty |
| Uremia | Dehydration | acids |
The frequency and form of presentation of encephalopathy in the same patient allows establishment of a predominant clinical course, within three possibilities:
- 1.
Episodic: considered a “recurrent encephalopathy” with precipitating factors or spontaneous delirium.
- 2.
Persistent: cognitive deficits, extra-pyramidal manifestations, sleep-pattern changes that cab be either mild or severe, but always continuous.
- 3.
Minimal: sub-clinical cases.
Hepatic encephalopathy is manifested in variable forms and can be associated with any neurological alteration, even with focal deficits. Frequently, there is cerebral edema that contributes to the clinical picture and increases the mortality in the patients with acute or chronic encephalopathy. The decrease in attention and changes of mental state can evolve to memory disorders, confusion, stupor and to coma; also, the varied combination of neurological signs include asterixis, rigidity, abnormal reflexes, Babinsky, and rarely, convulsions. One of the earliest signs of encephalopathy is an inversion in the sleep cycle.
Ammonium level is a key element in the diagnosis of hepatic encephalopathy, however its predictive value in cirrhotic patients is limited. Measuring the arterial levels and adjustment of the values according to the pH improve the predictive value, but these tests cannot be done in all hospitals. Recently, a relation between the levels of arterial ammonium and the development of brain herniation in acute hepatic failure has been demonstrated. Some other studies have shown that levels of more than 200 mg/dL of ammonium in hepatic encephalopathy stages III and IV are associated with cerebral herniation. However, other studies have shown no correlation between ammonium levels and the stage of hepatic encephalopathy.
Generally, the diagnosis can be done by exclusion. There is no specific alteration in the hepatic function tests; the presence of high levels of ammonium in an adequate clinical context suggests the diagnosis. However, this is only true for hepatic encephalopathy stages III and IV (possibly II), in which the values are presented with the objective signs described before. This is not true for stages I or II, where objective signs cannot be perceived, allowing progression of the underlying condition. Also, it has been demonstrated that ammonium levels do not correlate with the severity of encephalopathy or with the response to treatment.
For these reasons, imaging studies are suggested to diagnose encephalopathy stage I and stage II (or mild and minimal-persistent). Brain resonance imaging can demonstrate manganese deposits at the basal ganglia, showing a high resonant globus pallidus, as shown in figure 1. Computed tomography is used to examine the presence of atrophy or cerebral edema. Positron emission tomography can produce images reflecting biochemical or physiological processes. Finally, spectroscopy by resonance imaging makes evident the elevations in the spike of glutamine/glutamate and diminished myo-inositol and choline. This data can help treatment halt the progression of encephalopathy.
Certain neuropsychological tests have been approved by the majority of investigators but still require formal validation:
- 1.
Numeric connection A and B
- 2.
Line drawing
- 3.
Digital symbols and points following.
These types of tests have been named the PHES (Psychometric hepatic encephalopathy score) and can be done in ten minutes. The main objective is to evaluate the reaction time and the accusiocity, visual construction, concentration, attention and memory.
TreatmentThe treatment of patients with chronic hepatic encephalopathy includes establishing the therapeutic objectives. In recurrent encephalopathy the main objective is to avoid the acute episodes, while in the persistent form the objectives are toward improvement of chronic symptoms and quality of life. There are objective parameters that allow good characterization of the effects of treatment. One such parameter is the number of days that the patient’s autonomy is limited compared to his or her basal state (for example, whether he or she is able to work, walk, eat or clean him or herself without help). According to the optimal level for an individual patient, the degree of autonomy loss can be estimated (as an example: 25% not able to work, 50% not able to walk, 75% needs help to eat). There are tables developed to evaluate patients with dementia that can be useful in these circumstances (Barthel scale). In the best case, some psychometric tests and neuropsychological testing should also be done, so as to evaluate the effects of the chronic manifestations.
The treatment of hepatic encephalopathy should be accompanied by the treatment of the other complications of cirrhosis. The potential effects of the treatment should be considered against the risk of developing hepatic encephalopathy. In patients with ascitis, it is better to perform paracentesis or to use diuretics. When prescribing diuretics, they should be used in low doses that can be modified according to the response. It is useful to keep a record of the weight of the patient, the diuretic dose and the neurological manifestations. The patient and his or her family should understand that it is better to have mild edema than frequent development of encephalopathy. Between the different choices in the treatment of patients with gastrointestinal hemorrhage, endoscopic or pharmacological treatments are better options than the ones that increases the portal-systemic shunts (surgery or TIPS), because between 30 and 40% of the patients with TIPS can develop hepatic encephalopathy.
The development of hepatic encephalopathy is considered a sign of a bad prognosis. For this reason, patients who have presented with an episode of hepatic encephalopathy should be evaluated as candidates for hepatic transplantation.6 However, in cases of chronic persistent encephalopathy, the decision can be difficult. Dementia and other severe neurological signs such as paraplegia or psychiatric manifestations are considered irreversible. There are descriptions of some cases that show improvement of lesions after the transplant. The decision should be individualized for each patient.
One group of patients with chronic encephalopathy is characterized by the development of hepatic encephalopathy after the surgery for a portal-systemic shunt.
Part of the treatment objectives for patients with hepatic encephalopathy is the importance of applying some general measures to support the patient: nursing care, in selected cases the use of prophylactic endotracheal intubation, and during the period of altered mental state, proper nutritional support. Also, it is important to identify and treat the potential precipitating factors. In the context of gastrointestinal hemorrhage, searching for occult fecal blood or clinical evidence of hemorrhage, the detection of infections specifically of ascitis; additionally, correction of renal and electrolytes disturbances, avoiding the use of psychoactive medications such as benzodiazepines, narcotics and sedatives and preventing constipation with the use of laxatives.
Besides these measures, the patient should follow some dietary recommendations and receive non-absorbable disaccharide. In some cases, the use of neomycin, other antibiotics or dopaminergic drugs can be useful. However, none of these therapeutic measures has been evaluated with clinical trials of appropriate design and a significant number of patients. Accordingly, all of these measures have been criticized. However, clinical experience, together with the data presented in the scientific literature allows some recommendations about their use.
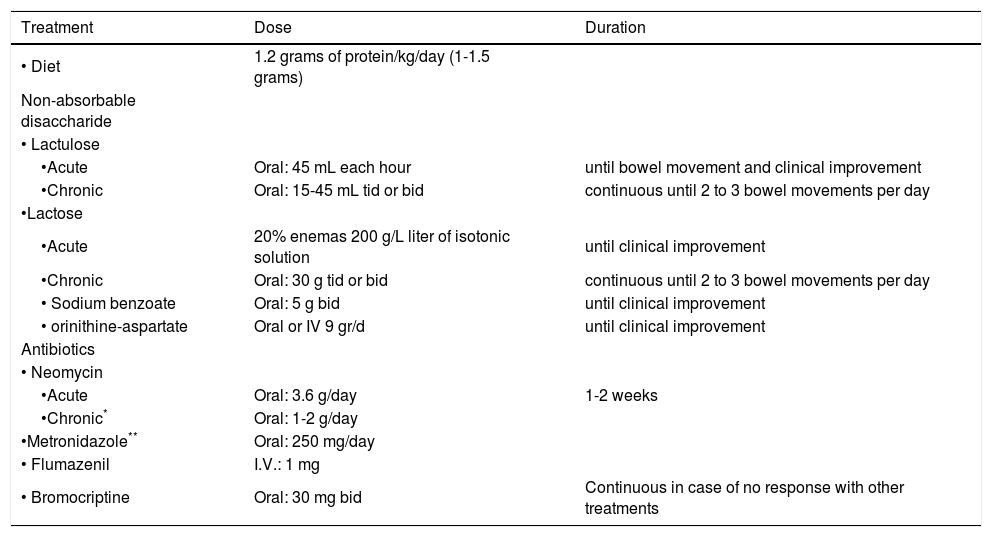
Treatment optionsIn table III the main measures of treatment for patients with hepatic encephalopathy are shown.
Treatment of hepatic encephalopathy.
| Treatment | Dose | Duration |
|---|---|---|
| • Diet | 1.2 grams of protein/kg/day (1-1.5 grams) | |
| Non-absorbable disaccharide | ||
| • Lactulose | ||
| •Acute | Oral: 45 mL each hour | until bowel movement and clinical improvement |
| •Chronic | Oral: 15-45 mL tid or bid | continuous until 2 to 3 bowel movements per day |
| •Lactose | ||
| •Acute | 20% enemas 200 g/L liter of isotonic solution | until clinical improvement |
| •Chronic | Oral: 30 g tid or bid | continuous until 2 to 3 bowel movements per day |
| • Sodium benzoate | Oral: 5 g bid | until clinical improvement |
| • orinithine-aspartate | Oral or IV 9 gr/d | until clinical improvement |
| Antibiotics | ||
| • Neomycin | ||
| •Acute | Oral: 3.6 g/day | 1-2 weeks |
| •Chronic* | Oral: 1-2 g/day | |
| •Metronidazole** | Oral: 250 mg/day | |
| • Flumazenil | I.V.: 1 mg | |
| • Bromocriptine | Oral: 30 mg bid | Continuous in case of no response with other treatments |
- 1.
Nutritional management
Classically, the recommendation for patients with encephalopathy is a low protein diet. However, there are few fundaments to support such a recommendation.8,9 It is noteworthy to consider that an important objective in the treatment of the encephalopathy is to diminish the plasma ammonium concentration. Oral intake of a high protein diet causes an increment in the post-prandial plasma ammonium; accordingly, it has been interpreted that a lower protein content diet may result in a lower ammoniac level. This interpretation may be wrong. In experimental models it has been observed that a free protein diet increases the ammonium concentration because of diminished activity of the enzymes in the urea cycle.10 It has been observed in stable cirrhosis that the protein diets cause a diminished urea synthesis.11
During an acute encephalopathy episode, the oral route of nutrition is usually avoided, and glucose parental solutions are administered.1 In most cases, the patient recovers in a few days and the oral route is again instituted. At this time, a moderate dose of protein (40 grams per day) is frequently administered and this is increased gradually until the maximum tolerance (70-80 grams per day). Probably, the protein restriction does not improve the course of acute encephalopathy. In studies that have evaluated the administration of branched amino acids, no difference was observed in the short-term development of encephalopathy according to the protein dose.8
A potential risk of protein restriction is a worsening of the nutritional status (usually bad in these patients), especially if the restriction is maintained for prolonged periods of time. In chronic hepatic encephalopathy, protein restriction can have negative effects on the patient, because the nutritional status is a parameter that improves the prognosis of cirrhosis significantly. Also, it is possible that an improvement in nutritional status is associated with better control of hepatic encephalopathy. An increase in muscle mass can facilitate the ammoniac metabolism by its transformation into glutamine.12
An improvement in nutritional status is difficult to achieve, but it has to be tried. It is considered that a high protein diet is necessary (1.2 g/kg/day) to reach a stable nitrogen balance. Both clinical experience and a classic study13 have shown that administration of high quantities of proteins can precipitate an encephalopathy episode and patients may be not able to tolerate it. It is therefore recommended to make progressive increases in proteins up to the maximum tolerance. In the case of development of encephalopathy, it is important to evaluate the possibility of other precipitating factors and to again try a progressive increase of proteins until the maximum tolerance. There is a formula for increasing tolerance by modifying the diet composition: namely, for the same quantity of nitrogen, vegetal proteins and milk derivatives develop less encephalopathy than does meat.14 In the case of vegetal proteins, it is believed that the benefit is due in part to the high fiber content.
Nutritional supplements rich in branch amino acids can be useful in some patients with chronic encephalopathy with poor tolerance to the diet proteins.9 Branch amino acid supplements have some anti-catabolic effects, maybe because of their energetic effects in the muscle, with this mechanism able to reduce the level of ammonium. In a study including 64 patients with cirrhosis and chronic hepatic encephalopathy, treatment with branched amino acids for six months was associated with fewer episodes of encephalopathy.15 However, other studies with fewer patients and of shorter duration have not found these results.16 An important aspect is that the supplements are expensive; they should be recommended in patients with poor nutritional status in whom there is no possibility of increasing the protein content of the diet because of the development of encephalopathy.
In general, in chronic hepatic encephalopathy it is recommended to administer a diet with sufficient proteins to maintain a good nitrogen balance (80 grams per day), but the quantity should be adapted to the nutritional status and tolerance to the high-protein-diet. It is better to administer many small meals to distribute the caloric content across five or six meals and to avoid prolonged periods of starvation. There is no scientific evidence that demonstrates that a low protein diet diminishes the number of encephalopathy episodes or improves the persistent neurological manifestations
- 2.
Non-absorbable disaccharide
Despite the lack of good clinical trials that demonstrate the efficacy of the non-absorbable disaccharides in the treatment of chronic hepatic encephalopathy, it is considered that they are useful for the treatment of the chronic symptoms and for the prevention of recurrences. There are two forms: lactulose (β-galactoside-fructose) and lactitol (β-galactoside-sorbitol). Its mechanism is dependent on a diminution in the plasma concentration of ammonium, an important factor in the development of encephalopathy.17 Their action is enabled by their metabolism by the colon bacterial flora, reached after their oral administration; in the bowel they are not absorbable because of the absence of specific dissacharidases. Also, they can be administered rectally in the form of enemas; this form of administration does not result in any improvement in the management of chronic illness.
The dose is administered orally two to three times per day, and it is very important to adjust the dose to enable two to three smooth bowel movements per day. It is not recommended to administer another laxative because it can confuse the clinical picture by giving the impression that an appropriate effect has been reached. The comparison in clinical trials between lactulose and lactitol has found similar efficacy and similar adverse effects.18 Both medications can produce flatus and abdominal discomfort. Also, there have been comparisons between the use of lactose, or lactitol plus either lactose or lactulose, with similar results and improvement of hepatic encephalopathy measured by the index of portal-systemic encephalopathy.19,25-28 It should be considered that they can cause diarrhea and dehydration, and both hydro and electrolyte imbalances should be reviewed, as they can worsen the encephalopathy. It is recommended to avoid administration of neomycin with a non-absorbable disaccharide.19,26 It may be necessary however to increase the dose of non-absorbable disaccharide with time, so as to maintain the effects obtained when their use commenced.
- 3.
Antibiotics
Neomycin and other antibiotics (metronidazole, vancomycin, rifaximin) are an alternative for patients intolerant to non-absorbable disaccharides, or in patients that are unresponsive to them. The mechanism of action is the decrease of ammoniac produced by the bowel flora. However, there are existing data that support that the position that the mechanism of neomycin action is by its effects on the metabolism of the intestinal mucosa.20 The preferred antibiotic is neomycin because it has been used more often.21 Neomycin has efficacy similar to lactulose in some clinical trials. The dose is 1 to 2 grams per day. Prolonged treatment with aminoglycosides or other antibiotics can be toxic. It is necessary to avoid its administration for more than six months and to be alert for signs of renal and auditory toxicity.
- 4.
Medications affecting neurotransmission Dopaminergic drugs were first introduced in the treatment of hepatic encephalopathy with the false neurotransmitter theory.22 This hypothesis is now obsolete. In spite of this fact, it is believed that in chronic hepatic encephalopathy, there is a deficit in the dopaminergic function resulting from manganese deposits in the basal ganglia.23 In most patients this dysfunction causes few symptoms; in some cases, however, it can cause a severe Parkinsonism, and these patients should also receive non-absorbable disaccharide. The dose of levodopa dose should be adjusted to the clinical response. It is important to note that dopaminergic drugs can cause constipation, so the dose of nonabsorbable disaccharide may be increased. Flumazenil and bromocriptine24 can have a direct brain effect by improving GABA-ergic pathways. It has been proposed that endogenous benzodiazepines can be present in patients with hepatic encephalopathy, causing inhibitory effects by its binding to GABAa receptors. Further, the administration of flumazenil can improve hepatic encephalopathy in approximately 15% of cases, but the results have not been of significant clinical benefit.
- 5.
Other therapies
The use of ornithine-aspartate provides substrates for the urea cycle (ornithine) and for the synthesis of glutamine (aspartate, by the transamination to glutamate), while also diminishing ammonium levels. It is available in oral or intravenous forms; however, the experience has demonstrated benefits in the treatment of acute and chronic encephalopathy. Sodium benzoate reduces the levels of ammonium by increasing urinary excretion. It reacts with glycine to form hypurato. For each molecule of benzoate the kidneys excrete a mole of hydrogen. The drug is not expensive compared with lactulose, and its effects are comparable.
- 6.
Management of splanchnic circulation
The large gastro-renal or spleno-renal communications have been associated with spontaneous encephalopathy episodes. The occlusion of this systemic collateral can result in improvement of hepatic encephalopathy; however, this should be performed in a center with experience and after all the commented measures have failed to control hepatic encephalopathy. The presence of bio-artificial livers has been effective not only in supporting hepatic function but also in the improvement of neurological function, specifically in the improvement of cerebral pressure, which is elevated in the majority of these patients. Recently, dialysis with absorbent membranes (MARS) is being studied in the United States,30,31 with the results to be presented shortly.
The latest therapy for hepatic encephalopathy is orthotopic liver transplantation. The frequency and severity of the disease are important factors in determining the candidates.