Background and relationale for the study. Genome-wide association studies have identified host genetic variation to be critical for spontaneous clearance and treatment response in patients infected with hepatitis C virus. Recently, the role of the IFNL3 polymorphisms in influencing the spontaneous clearance of HCV, the response to interferon and the progression of liver fibrosis, was also demonstrated in patients with thalassemia major infected by genotype 1b. In the present study we retrospectively analyzed 368 anti-HCV positive patients with beta-thalassemia at two Italian major centers in Cagliari and Torino.
Results. C/C variant of polymorphism rs12979860 was related to response to interferon treatment and, above all, to spontaneous clearance of the virus. However, the positive predictive power was stronger for viral persistence than spontaneous clearance and in such respect the TT allele was more predictive than CC. The methylation associated polymorphism rs4803221 had independent effects with respect to rs12979860 and the haplotype tagged by SNP rs12979860 and rs4803221 significantly could improve the viral clearance prediction in infected patients. Neither necroinflammation or bilirubin values in the chronic phase of the hepatitis C were related to IFNL3 polymorphisms. No relation among IFNL3 polymorphisms and fibrosis stage directly shown by the liver biopsy was found.
Conclusions. Also in thalassemia the SNPs on chromosome 19q13 closely associates with spontaneous and treatment-induced HCV clearance. The viral clearance prediction is significantly improved by the haplotype tagged by SNP rs12979860 and rs4803221. Neither necroinflammation, bilirubin values or fibrosis stage seem to be related to IFNL3 polymorphisms.
Genetic testing [genome-wide association studies (GWAS)] have recently identified host genetic variation to be critical for spontaneous clearance and treatment response in patients infected with hepatitis C virus (HCV).1–5 Compared with all other baseline host and viral variables, different polymorphisms of the interleukin-28B (IFNL3) gene, situated on chromosome 19, have been reported as the strongest predictor of HCV therapy response and spontaneous viral clearance. In this respect, the rs1297860 C and the rs8099917 T variants, located 3 and 8 kb upstream of the IFNL3 gene, which have some level of linkage disequilibrium (R2-0.50 in Caucasians), seem to be the two strongest genetic predictors. Beyond their identification, little is known about the mechanisms involved between these genomic variants and viral clearance and it is still uncertain whether these polymorphisms play a causal role. IFNL3 encodes IFN-λ3, which induces antiviral activity by itself and through the Janus kinase-signal transducer and activator of transcription (JAK-STAT) complex, which induces IFN-stimulated genes (ISGs) that also have antiviral activity against HCV. It has been shown that unfavourable IFNL3 genetic variations are associated with higher pre-activated levels of ISGs, which could explain the more frequent chronicization and poor response to antiviral treatment in these patients.6,7 Data on the influence of the IFNL3 gene polymorphisms on the natural history of untreated chronic HCV infection are scarce. The correlation between IFNL3 polymorphisms and progression of liver fibrosis is still controversial. Several studies suggest that the presence of rs8099917 T/T genotypes is associated to necroinflammation, and in others the T allele was found to be an independent predictor of severe liver fibrosis and hepatocarcinoma.8–11 In other reports, on the contrary, genetic variation in the IFNL3 gene are not associated with fibrosis progression in patients with chronic hepatitis C.12–14 Interestingly, Tillman, et al. have reported that jaundice during acute hepatitis C is more common among patients with rs12979860 C/C genotype than non-C/C patients and that women with the C/T or T/T genotype who did not develop jaundice have a lower chance of spontaneous clearance of HCV infection.15
It is unknown whether rs12979860 and rs8099917 exert direct biological effects or are in linkage disequilibrium with other functional polymorphisms. Several investigators have performed gene mapping failing to detect new single nucleotide polymorphisms (SNPs) with a stronger genetic effect or with a clear functional mechanism.16 Other studies, however, have demonstrated that methylation associated polymorphisms (that is polymorphisms associated with a change of methylation, e.g. a loss or a gain of methylation) located within the 5’ region upstream from the transcription start of the IFNL3/A locus are better predictors of spontaneous and treatment-induced clearance of hepatitis C infection than polymorphisms previously described.17 Among them, rs4803221 is a C to G (C/G) polymorphism resulting in a loss of methylation, located -500 bp upstream rs12979860 which has been associated with reduced clearance and response to treatment.
Recently, the role of the IFNL3 polymorphisms in influencing the spontaneous clearance of HCV, the response to interferon and the progression of liver fibrosis, was also demonstrated in patients with thalassemia major infected by genotype 1b.18
Although hepatitis C is no longer a major threat in the European blood supply,19 these findings are relevant because HCV acquired through transfusion before the implementation of blood donor screening, remains one of the most important problems among patients with thalassemia, given the reported high presence of HCV antibodies in multitransfused patients and a noteworthy prevalence of clinically significant fibrosis.20,21 Moreover, hepatocarcinoma is becoming frequent with the ageing population of patients with thalassemia.22
Material and MethodsIn the present study we retrospectively analyzed 583 patients with beta-thalassemia at two Italian major centers in Cagliari and Torino. Two hundred and fifteen were anti-HCV negative and 368 anti-HCV positive by ELISA. One hundred twenty-two patients had started red cell transfusions after the implementation of blood donor screening in 1991 (4 of them were infected by HCV) and 461 before (364 infected). Among 368 anti-HCV positive patients 149 (40.5%) had spontaneously cleared the virus and 219 (59.5%) were chronically infected (at least a biannual determination by real time PCR).
Viral genotype was known in 215 patients. One hundred thirty-three subjects with chronic infection had been infected by severe genotypes (1 or 4) and 83 by side genotypes (2, 3 or 5). Ninety four HCVRNA positive patients had undergone liver biopsy and none of them had received antiviral therapy before. Mean age at the time of liver biopsy was 20 ± 5 years.
Fifty patients had been treated with Interferon in the ‘90s, of whom 20 presented sustained virological response (SVR) and 30 presented non sustained virological response (NSVR). Since 2010, most patients with chronic persistent HCV infection received Peg-interferon and ribavirin, but, having only 27 of them completed the treatment since at least 6 months, the correlation between IFNL3 polymorphisms and response to this kind of treatment could not be evaluated due to the low number.
The 368 anti-HCV positive patients were genotyped at the polymorphic sites on chromosome 19. The relationship between IFNL3 variants, liver necroinflammation, and fibrosis was evaluated. Although the bilirubin values during the acute phase of hepatitis C were not available, four values per year until 2010 for the last 20 years per patient (or until the antiviral treatment) were recorded by mean of Webthal®, a Web-Based Multicentric Database, as well as ALT and serum ferritin values measured at the same time. The use of Webthal for the clinical follow-up of the patients and for scientific purposes was approved by the Ethics Committees of the two hospitals. All patients registered in Webthal signed informed consent to the use of their clinical data for research studies and objectives. Moreover, each patient, and their parents if minor, signed a written consent for DNA testing.
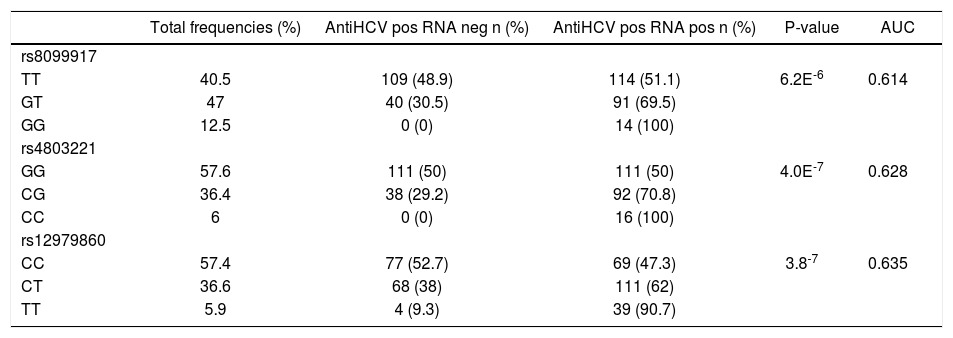
Three SNPs, rs12979860, rs8099917 and rs4803221 were genotyped. DNA was extracted from venous peripheral blood with standard methods while SNPs were directly genotyped using ABI TaqMan assay (Applied Biosystems, Warrington, UK). SNP details as well as genotype frequency measures are reported in table 1.
Genotype frequencies of IFNL3 polymorphisms in 368 subjects with beta thalassemia and antiHCV antibodies.
| Total frequencies (%) | AntiHCV pos RNA neg n (%) | AntiHCV pos RNA pos n (%) | P-value | AUC | |
|---|---|---|---|---|---|
| rs8099917 | |||||
| TT | 40.5 | 109 (48.9) | 114 (51.1) | 6.2E-6 | 0.614 |
| GT | 47 | 40 (30.5) | 91 (69.5) | ||
| GG | 12.5 | 0 (0) | 14 (100) | ||
| rs4803221 | |||||
| GG | 57.6 | 111 (50) | 111 (50) | 4.0E-7 | 0.628 |
| CG | 36.4 | 38 (29.2) | 92 (70.8) | ||
| CC | 6 | 0 (0) | 16 (100) | ||
| rs12979860 | |||||
| CC | 57.4 | 77 (52.7) | 69 (47.3) | 3.8-7 | 0.635 |
| CT | 36.6 | 68 (38) | 111 (62) | ||
| TT | 5.9 | 4 (9.3) | 39 (90.7) |
The frequencies were calculated in the whole group and, separately, in HCVRNA positive and HCVRNA negative patients. AUC: area under the curve.
Frequencies of both SNPs and haplotypic pairs in patients chronically infected and in those who cleared the virus were analyzed. SNPs data were phased using PHASE software (Stephens, M 2003) and kept when phasing probability was higher than 0.9. Differences between groups were tested using genotypic test as implemented in pLink software and Mann-Whitney U-test upon data characteristics.
Furthermore, to check whether SNPs or haplotypes frequencies were associated with spontaneous viral response while controlling for confounders, a binary logistic regression model was developed. Goodness of fit of the model was assessed through Hosmer & Lemeshow test while Nagelkerke R2 was used to measure how useful explanatory variables were in predicting the outcome. Finally, predictive ability of SNPs and haplotypes were assessed by calculating the area under receiver operating characteristics (ROC) curves. All genetic analysis were performed using the PLINK software package, version 1.07 (Purcell, et al., 2007) while the SPSS statistical software package, version 18.00 (SPSS, IBM, Somers, NY, USA), was used for subsequent analysis, using a critical alpha of 0.05. Patient characteristics were described as relative frequencies or median with 5th and 95th percentile values.
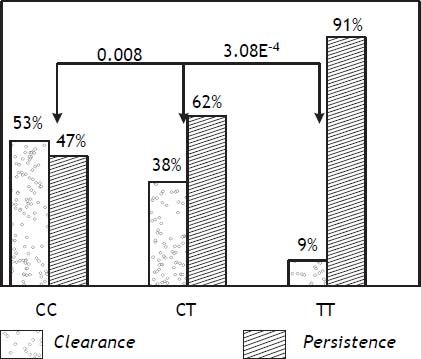
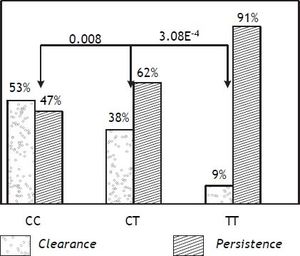
ResultsAs shown in table 1, rs12979860 and rs4803221 were the polymorphisms more associated with viral clearance and persistence (p = 3.8E-7 and p = 4.0E-7 respectively). The positive predictive power (PPP) of rs12979860 was stronger for viral persistence than for spontaneous clearance (PPP = 0.91) and in such respect it corresponded to a recessive model for the T allele (p = 3.8E-4 for TT vs. CT genotype while p = 0.08 for CT vs. CC genotype). On the other hand, a dominant model for the C allele of rs12979860 was not alike predictive of viral clearance (PPP = 0.52) (Figure 1).
Positive predictive power of rs12979860 polymorphisms in respect of viral persistence and of spontaneous clearance. The positive predictive power (PPP) of rs12979860 was stronger for viral persistence than for spontaneous clearance and a recessive model for the T allele was the most predictive of viral persistance.
The polymorphism rs8099917, was observed to have a high linkage disequilibrium with rs4803221 (R2 = 0.9), while rs1297860 showed low linkage disequilibrium with rs8099917 or rs4803221 (R2 = 0.43 and 0.47 respectively).
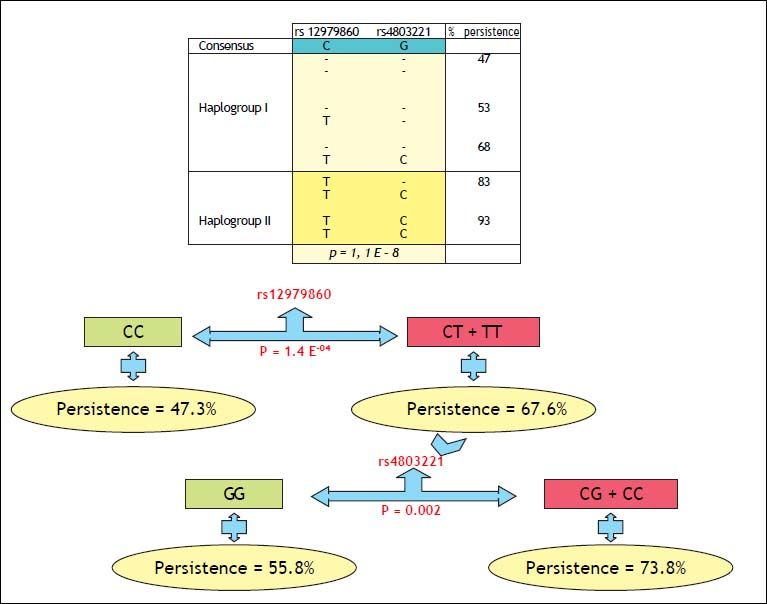
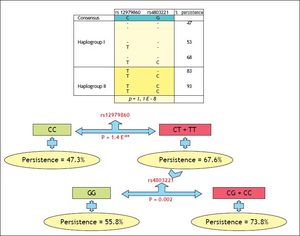
Binary logistic regression analysis, including rs12979860, rs4803221, gender and UGT1A1, showed that not categorized rs4803221 as well as genotype TT of rs12979860 were associated with viral persistence (OR = 2.36 p = 0.007 and OR = 6.04 p = 0.02 respectively). Rs8099917 was not included in the model for its almost complete linkage disequilibrium with rs4803221.
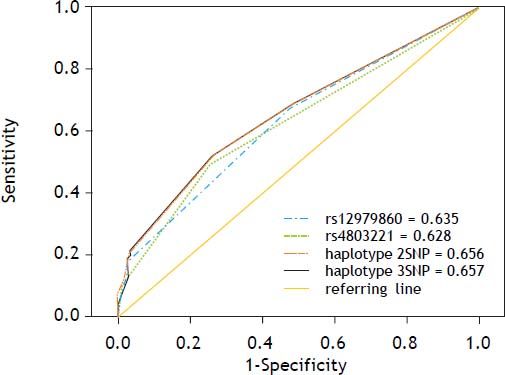
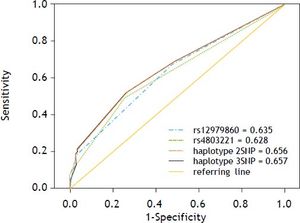
Predictive ability of haplotypes formed by rs4803221 and rs12979860, as well as haplotypes formed by all three SNPs, compared to predictive ability of the best predictor single SNP (rs12979860) using ROC curve analysis, showed that the haplotype including rs4803221 and rs12979860 was only slightly less predictive than haplotype including all the three SNPs (AUC = 0.656 and AUC = 0.657 respectively). On the other hand, the haplotype including rs4803221 and rs12979860 outperformed rs12979860 in predicting viral clearance (AUC = 0.635) (Figures 2 and 3).
As expected, patients with chronic persistent HCV infection had mean ALT values of the last 22 years significantly higher than patients who had cleared the virus (91.7 ± 57.8 IU/L vs. 46.6 ± 27.2 IU/L, p = 3.260E-17). However, among HCVRNA positive patients, mean ALT values were not related to the rs12979860 and rs4803221 polymorphisms (p = 0.76 as for rs12979860 and p = 0.66 as for rs4803221). On the contrary, mean ALT were significantly related to mean serum ferritin values both in patients with chronic infection and in those who cleared the virus (p = 1.25E-09 and 2.58E-03, respectively) and serum ferritin was not statistically different in the two groups of patients (1,990 ± 1,290 ng/mL vs. 2,020 ± 1,440 mg/mL, p = 0.984). Mean total bilirubin values were not statistically different between HCVRNA positive and negative patients (31 ± 29 μmol/L vs. 29 ± 27 μmol/L, p = 0.086), and, among HCVRNA positive, with respect to the rs12979860 and rs4803221 polymorphisms (p = 0.14 and 0.09, respectively).
UGT1A1 polymorphisms were strongly related to the bilirubin values both considering all patients (mean bilirubin 25 ± 25 μmol/L in TA6/TA6, 28.2 ± 15 μmol/L in TA6/TA7 and 56 ± 28 μmol/L in TA7/ TA7 patients, p = 8.60E-14) and patients with chronic infection (mean bilirubin 27 ± 31 μmol/L in TA6/TA6, 29 ± 15 μmol/L in TA6/TA7 and 61 ± 30 μmol/L in TA7/TA7 patients, p = 2.09E-08).
By the Desmet score, 16 patients (17%) had no fibrosis, 35 (37.2%) had mild fibrosis (F1), 24 (25.5%) moderate fibrosis (F2), 17 (18.1%) sever fibrosis (F3) and 2 (F4) had cirrhosis.23
Rs12979860 C/C genotype was present in 24 patients with F0-F2 and 9 patients with F3-F4, C/T in 46 patients with F0-F2 and 8 patients with F3-F4 and T/T in 5 patients with F3-F4 and 2 patients with F3-F4 (p = 0.26). As for rs4803221, G/G genotype was present in 37 patients with F0-F2 and 11 patients with F3-F4, C/G in 34 patients with F0-F2 and 8 patients with F3-F4 and T/T in 4 patients with F3-F4 and no patients with F3-F4 (p = 0.75).
However, the present study is underpowered to observe clinically relevant differences in proportions, indeed we only have 20% power to observe a 10% difference between group F0-F2 and F3-F4 with the present sample size.
Rs12979860 and rs4803221 were also associated with SVR and NSVR (p < 0.01 and p = 0.04 respectively). Among 50 patients treated with interferon, 20 achieved a SVR. None of them carried the T/T genotype in the rs1297860 while 13 were C/C. Viceversa, among 30 patients with persistent HCV viremia (NSVR), 7 carried T/T genotype. rs4803221 had similar properties, with 6 NSVR patients carrying recessive genotype (C/C) vs. no one in SVR, and with a frequency of 50% in dominant genotype with 14 out of 20 patients who achieved a SVR.
DiscussionThis study confirms that, in thalassemia patients as in general population, the SNPs on chromosome 19q13 closely associates with the natural course and treatment response of chronic hepatitis C. As in a recent paper, indeed, also in our population, CC variant of polymorphism rs12979860 was related to response to interferon treatment and, above all, to spontaneous clearance of the virus.18 However, it seems noteworthy to underline that, as for rs12979860, the positive predictive power was stronger for viral persistence than spontaneous clearance and in such respect the TT allele was more predictive than CC. Moreover, in our population, while rs8099917 has an almost complete LD with rs4803221, the methylation associated polymorphism rs4803221 has independent effects with respect to rs12979860. This finding led us to develop an algorithm that, considering rs4803221, significantly may improve the viral clearance prediction in patients presenting the T allele at rs12979860. Recently, the same rs4803221 was found to predict failure to respond to antiviral therapy better than rs8099917 and rs12979860 in 199 treated patients infected by virus C genotype 1.17
Since both the variants on SNP rs12979860 and rs4803221 remove a CpG site located within a CpG island, erasing two potential methylation sites, it is believable the hypothesis that, this could lead to a minor resistance to unfolding and an increased expression of IFNL3 and a down regulation of interferon sensitive genes resulting in a reduced innate antiviral activity and poor response to therapy. Further studies are needed to demonstrate that the haplotype tagged by SNP rs12979860 and rs4803221 is the major causative.17
Although we could not evaluate the correlation between IFNL3 polymorphisms and pegylated interferon plus ribavirin, currently considered the standard of care for non-genotype 1 chronic hepatitis C and whether the correlations found in patients with hepatitis C genotype 1 may be found in patients with other genotypes, our study sheds light on other, new aspects of the influence of the IFNL3 polymorphisms on natural history of hepatitis C in patients with beta thalassemia. First, we have demonstrated that in thalassemia patients the rs12979860 as the rs4803221 polymorphisms are not associated with necroinflammation, differently from that reported by Agundez, et al. and Thompson, et al. who have found in general population that rs12979860C/C genotype is associated with higher serum ALT than the remaining genotypes and by Abe, et al. who found that ALT levels were lower in carrier of the rs8099917 T/T genotype.13,14,24 This difference could be due to the confounding effect of liver iron, which as a primary role in inducing liver damage in subjects with thalassemia, as demonstrated by the highly significant relation between mean ALT and mean serum ferritin values in our patients.25 Tillman reported that the jaundice during acute hepatitis C is more common among patients with rs12979860 C/C genotype than with other variants and that in non-C/C patients, jaundice is associated with a higher likelihood of spontaneous clearance compared with those without jaundice.15 Although we could not confirm or deny these findings, no correlation was found between UGT1A1 polymorphisms and persistence of the virus. In addition, the total bilirubin long follow-up allowed us to demonstrate for the first time that, at least in patients with beta thalassemia, there is no correlation between rs12979860 polymorphisms and bilirubin values, which are confirmed to be strictly dependent on the UGT1A1 polymorphisms.26,27
The relation between IFNL3 polymorphism and the stage of fibrosis is controversial. Di Marco, et al. reported that the carrier state of the minor alleles at rs12979860 and rs8099917 sites were associated with more severe liver fibrosis in a group of 131 patients with thalassemia major and chronic HCV infection.18 In general population, several authors found higher fibrosis among carriers of the rs8099917TT genotype.9–11 Nevertheless, Thompson, et al. reported that the rs12979860 polymorphism was not associated with advanced hepatic fibrosis in patients with virus C chronic hepatitis, and Agimdez, et al. did not find any relation among the IFNL3 polymorphisms and fibrosis stage directly shown by the liver biopsy.13,14 These findings are in agreement with our results. Even though the number of patients undergone liver biopsy in our study is smaller than those examined by Di Marco, et al., the lack of association between necroinflammation and IFNL3 polymorphisms and the previously demonstrated significant correlation between rate of fibrosis progression and hepatic iron concentration in HCV-positive patients with thalassemia could explain and support these observations.25
In conclusion, also in thalassemia the SNPs on chromosome 19q13 closely associates with spontaneous and treatment-induced HCV clearance. The haplotype tagged by SNP rs12979860 and rs4803221 significantly could improve the viral clearance prediction in infected patients. Neither necroinflammation, bilirubin values in the chronic phase of the hepatitis C or fibrosis stage are related to IFNL3 polymorphisms.
Noteworthy, the new dinucleotide variant ss469415590 (TT or AG), located upstream of IFNL3 and in high linkage disequilibrium with rs12979860, has been recently discovered and demonstrated strongly associated with HCV clearance in individuals of African ancestry, but also in Europeans and Asians.28 Further studies are needed to evaluate if this variant has a role in the genetic regulation of HCV clearance in patients with beta thalassemia.