Previous studies have identified treatment disparities in the treatment of hepatocellular carcinoma (HCC) based on insurance status and provider. Recent studies have shown more Americans have healthcare insurance; therefore we aim to determine if treatment disparities based on insurance providers continue to exist.
Materials and methodsA retrospective database analysis using the NIS was performed between 2010 and 2013 including adult patients with a primary diagnosis of HCC determined by ICD-9 codes. Multivariable logistic regressions were performed to analyze differences in treatment, mortality, features of decompensation, and metastatic disease based on the patient's primary payer.
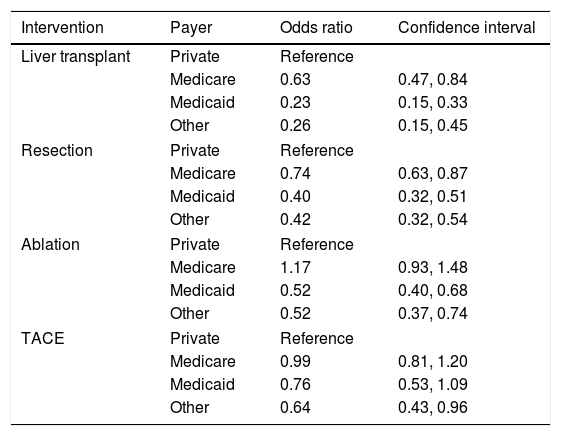
ResultsThis study included 62,368 patients. Medicare represented 44% of the total patients followed by private insurance (27%), Medicaid (19%), and other payers (10%). Patients with Medicare, Medicaid, and other payer were less likely to undergo liver transplantation [(OR 0.63, 95% CI 0.47–0.84), (OR 0.23, 95% CI 0.15–0.33), (OR 0.26, 95% CI 0.15–0.45)] and surgical resection [(OR 0.74, 95% CI 0.63–0.87), (OR 0.40, 95% CI 0.32–0.51), (OR 0.42, 95% CI 0.32–0.54)] than patients with private insurance. Medicaid patients were less likely to undergo ablation then patients with private insurance (OR 0.52, 95% CI 0.40–0.68). Patients with other forms of insurance were less likely to undergo transarterial chemoembolization (TACE) compared to private insurance (OR 0.64, 95% CI 0.43–0.96).
ConclusionInsurance status impacts treatment for HCC. Patients with private insurance are more likely to undergo curative therapies of liver transplantation and surgical resection compared to patients with government funded insurance.
The incidence of hepatocellular carcinoma (HCC) has exponentially increased over the last decade with greater than 25,000 patients diagnosed in 2014 [1,2]. Unlike many other malignancies, the mortality rate is also increasing at an average of 2% a year and the 5 year survival rate remains less than 20% [2]. HCC is quickly becoming one of the leading causes of cancer related mortality in the United States despite advancements in treatment with liver transplantation, surgical resection, ablation, and transarterial chemoembolization (TACE) [3–5].
With the growing attention to the need for available and affordable healthcare in the United States, more citizens had some form of insurance in 2014 compared to 2010, according to a recent government census. The uninsured rate dropped from 16% to 10%; however this represents 33 million American citizens still without healthcare insurance [6]. Despite more Americans having insurance, disparities in treatment based on insurance payer continue to exist and HCC is not an exception. Previous studies on hepatocellular carcinoma (HCC) showed that patients with Medicaid, Medicare, or no insurance were less likely to undergo surgery for early stage HCC compared to patients with private insurance. In the instances where surgery was completed, a higher rate of complications was noted in patients who did not have private insurance [7].
With the increasing incidence and worsening mortality in HCC, it becomes crucial to reevaluate disparities in treatment for HCC based on insurance provider given of the effects on HCC on healthcare costs, morbidity, and mortality.
We hypothesized that patients with Medicaid, Medicare, and other forms of insurance, including self-pay, no charge, Workers Compensation, CHAMPUS, CHAMPVA, Title V, and other government programs will be less likely to receive curative treatment for HCC than patients with private insurance.
2Materials and methodsData source: A retrospective database analysis using the Nationwide Inpatient Sample (NIS) was performed between 2010 and 2013. The NIS is part of the Healthcare Cost and Utilization Project (HCUP). This database represents information from over 7 million hospital discharges annually from hospitals across the United States and therefore is one of the largest, publically available databases. Information obtained from this database includes primary and secondary diagnoses, procedures, and demographic information [8]. This information is de-identified to protect the privacy of the patients, the physician involved in care, and the hospital in which care was received; therefore this study is exempt from review by The Ohio State University Institutional Review Board (IRB).
Sample study: Patients were identified using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9) code for HCC (155.0). Patients were excluded from this study if they were under the age of 18 or if they had cancer in the liver that was non-hepatic in origin. Patients were evaluated based on their insurance provider, which was defined as Medicaid, Medicare, private insurance and other which includes self-pay, no charge, Workers Compensation, CHAMPUS, CHAMPVA, Title V, and other government programs.
Outcomes of interest: The primary outcome of interest included evaluation of treatment disparities based on insurance provider. Treatment for HCC included liver transplantation, surgical resection, ablation, TACE, and noninvasive which includes patients that did not receive treatment or treatment was not coded. Secondary outcomes of interest included difference in features of liver decompensation, metastatic disease and inpatient mortality. Features of liver decompensation were defined as the presence of ascites, coagulopathy, esophageal varices, portal hypertension gastropathy, encephalopathy, edema, hepatorenal syndrome, and spontaneous bacterial peritonitis. In our analysis, feature of liver decompensation and metastatic disease were represented as categorical variables in Table 1 and dichotomous variables in Table 2 for the multivariable analysis.
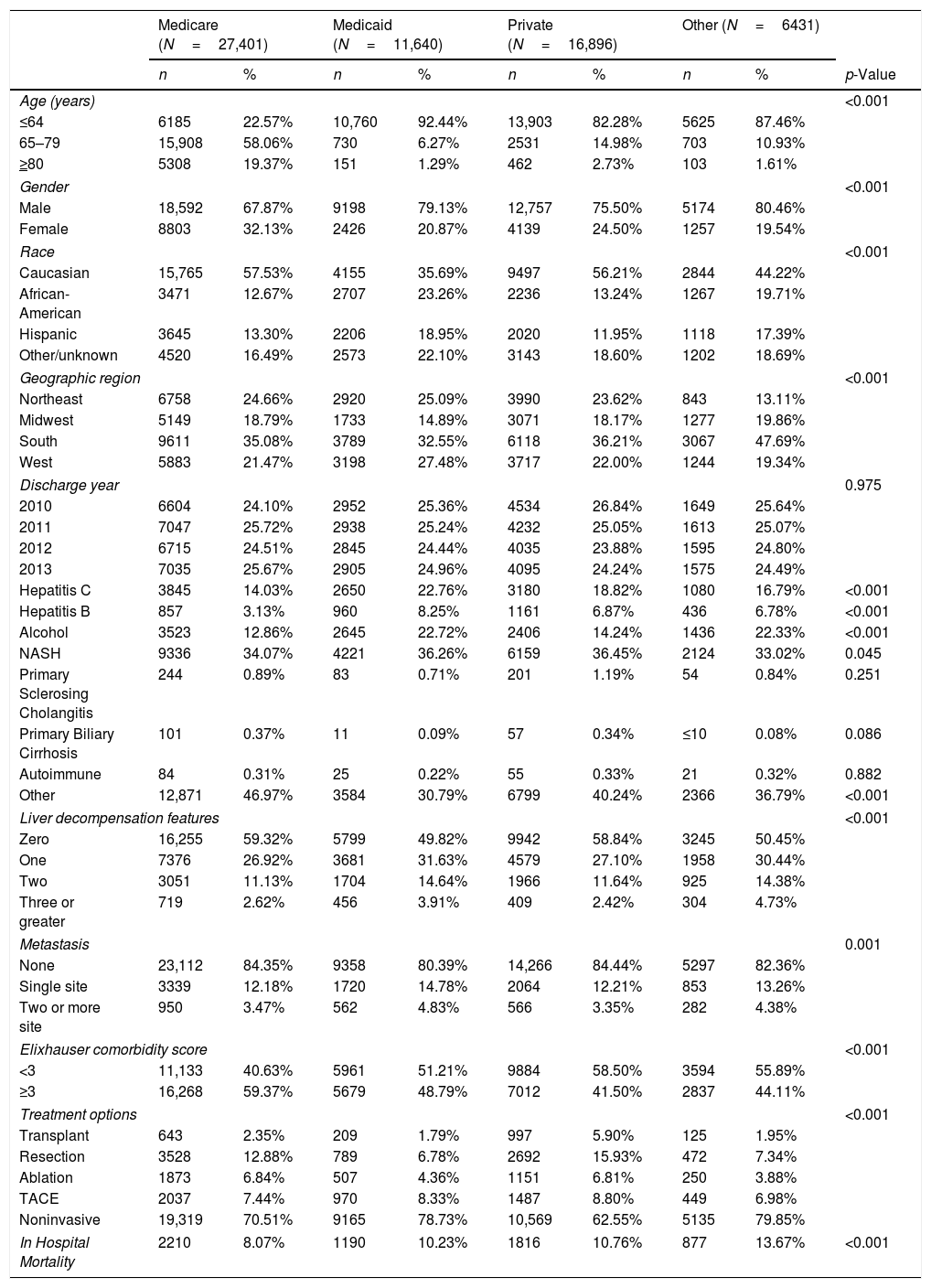
Demographic and clinical parameters in patients with hepatocellular carcinoma by primary payer.
| Medicare (N=27,401) | Medicaid (N=11,640) | Private (N=16,896) | Other (N=6431) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p-Value | |
| Age (years) | <0.001 | ||||||||
| ≤64 | 6185 | 22.57% | 10,760 | 92.44% | 13,903 | 82.28% | 5625 | 87.46% | |
| 65–79 | 15,908 | 58.06% | 730 | 6.27% | 2531 | 14.98% | 703 | 10.93% | |
| ≥80 | 5308 | 19.37% | 151 | 1.29% | 462 | 2.73% | 103 | 1.61% | |
| Gender | <0.001 | ||||||||
| Male | 18,592 | 67.87% | 9198 | 79.13% | 12,757 | 75.50% | 5174 | 80.46% | |
| Female | 8803 | 32.13% | 2426 | 20.87% | 4139 | 24.50% | 1257 | 19.54% | |
| Race | <0.001 | ||||||||
| Caucasian | 15,765 | 57.53% | 4155 | 35.69% | 9497 | 56.21% | 2844 | 44.22% | |
| African-American | 3471 | 12.67% | 2707 | 23.26% | 2236 | 13.24% | 1267 | 19.71% | |
| Hispanic | 3645 | 13.30% | 2206 | 18.95% | 2020 | 11.95% | 1118 | 17.39% | |
| Other/unknown | 4520 | 16.49% | 2573 | 22.10% | 3143 | 18.60% | 1202 | 18.69% | |
| Geographic region | <0.001 | ||||||||
| Northeast | 6758 | 24.66% | 2920 | 25.09% | 3990 | 23.62% | 843 | 13.11% | |
| Midwest | 5149 | 18.79% | 1733 | 14.89% | 3071 | 18.17% | 1277 | 19.86% | |
| South | 9611 | 35.08% | 3789 | 32.55% | 6118 | 36.21% | 3067 | 47.69% | |
| West | 5883 | 21.47% | 3198 | 27.48% | 3717 | 22.00% | 1244 | 19.34% | |
| Discharge year | 0.975 | ||||||||
| 2010 | 6604 | 24.10% | 2952 | 25.36% | 4534 | 26.84% | 1649 | 25.64% | |
| 2011 | 7047 | 25.72% | 2938 | 25.24% | 4232 | 25.05% | 1613 | 25.07% | |
| 2012 | 6715 | 24.51% | 2845 | 24.44% | 4035 | 23.88% | 1595 | 24.80% | |
| 2013 | 7035 | 25.67% | 2905 | 24.96% | 4095 | 24.24% | 1575 | 24.49% | |
| Hepatitis C | 3845 | 14.03% | 2650 | 22.76% | 3180 | 18.82% | 1080 | 16.79% | <0.001 |
| Hepatitis B | 857 | 3.13% | 960 | 8.25% | 1161 | 6.87% | 436 | 6.78% | <0.001 |
| Alcohol | 3523 | 12.86% | 2645 | 22.72% | 2406 | 14.24% | 1436 | 22.33% | <0.001 |
| NASH | 9336 | 34.07% | 4221 | 36.26% | 6159 | 36.45% | 2124 | 33.02% | 0.045 |
| Primary Sclerosing Cholangitis | 244 | 0.89% | 83 | 0.71% | 201 | 1.19% | 54 | 0.84% | 0.251 |
| Primary Biliary Cirrhosis | 101 | 0.37% | 11 | 0.09% | 57 | 0.34% | ≤10 | 0.08% | 0.086 |
| Autoimmune | 84 | 0.31% | 25 | 0.22% | 55 | 0.33% | 21 | 0.32% | 0.882 |
| Other | 12,871 | 46.97% | 3584 | 30.79% | 6799 | 40.24% | 2366 | 36.79% | <0.001 |
| Liver decompensation features | <0.001 | ||||||||
| Zero | 16,255 | 59.32% | 5799 | 49.82% | 9942 | 58.84% | 3245 | 50.45% | |
| One | 7376 | 26.92% | 3681 | 31.63% | 4579 | 27.10% | 1958 | 30.44% | |
| Two | 3051 | 11.13% | 1704 | 14.64% | 1966 | 11.64% | 925 | 14.38% | |
| Three or greater | 719 | 2.62% | 456 | 3.91% | 409 | 2.42% | 304 | 4.73% | |
| Metastasis | 0.001 | ||||||||
| None | 23,112 | 84.35% | 9358 | 80.39% | 14,266 | 84.44% | 5297 | 82.36% | |
| Single site | 3339 | 12.18% | 1720 | 14.78% | 2064 | 12.21% | 853 | 13.26% | |
| Two or more site | 950 | 3.47% | 562 | 4.83% | 566 | 3.35% | 282 | 4.38% | |
| Elixhauser comorbidity score | <0.001 | ||||||||
| <3 | 11,133 | 40.63% | 5961 | 51.21% | 9884 | 58.50% | 3594 | 55.89% | |
| ≥3 | 16,268 | 59.37% | 5679 | 48.79% | 7012 | 41.50% | 2837 | 44.11% | |
| Treatment options | <0.001 | ||||||||
| Transplant | 643 | 2.35% | 209 | 1.79% | 997 | 5.90% | 125 | 1.95% | |
| Resection | 3528 | 12.88% | 789 | 6.78% | 2692 | 15.93% | 472 | 7.34% | |
| Ablation | 1873 | 6.84% | 507 | 4.36% | 1151 | 6.81% | 250 | 3.88% | |
| TACE | 2037 | 7.44% | 970 | 8.33% | 1487 | 8.80% | 449 | 6.98% | |
| Noninvasive | 19,319 | 70.51% | 9165 | 78.73% | 10,569 | 62.55% | 5135 | 79.85% | |
| In Hospital Mortality | 2210 | 8.07% | 1190 | 10.23% | 1816 | 10.76% | 877 | 13.67% | <0.001 |
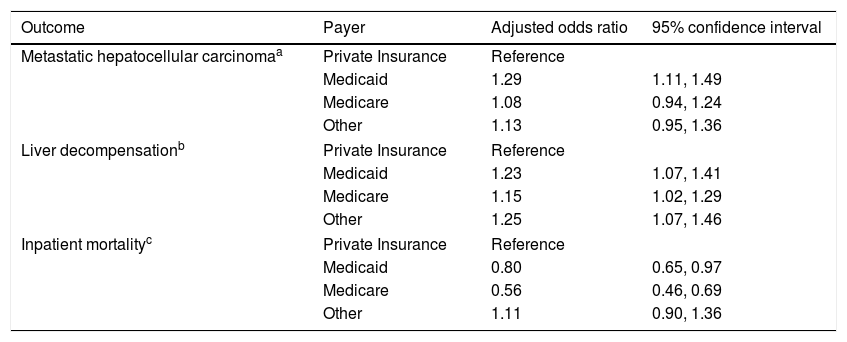
Multivariable logistic regressions comparing outcomes of hepatocellular carcinoma by payer.
| Outcome | Payer | Adjusted odds ratio | 95% confidence interval |
|---|---|---|---|
| Metastatic hepatocellular carcinomaa | Private Insurance | Reference | |
| Medicaid | 1.29 | 1.11, 1.49 | |
| Medicare | 1.08 | 0.94, 1.24 | |
| Other | 1.13 | 0.95, 1.36 | |
| Liver decompensationb | Private Insurance | Reference | |
| Medicaid | 1.23 | 1.07, 1.41 | |
| Medicare | 1.15 | 1.02, 1.29 | |
| Other | 1.25 | 1.07, 1.46 | |
| Inpatient mortalityc | Private Insurance | Reference | |
| Medicaid | 0.80 | 0.65, 0.97 | |
| Medicare | 0.56 | 0.46, 0.69 | |
| Other | 1.11 | 0.90, 1.36 | |
Model is adjusted for age, gender, race, geographic region, hepatitis C, alcohol, NASH, liver decompensation features, and Elixhauser comorbidity score.
Covariates: Multiple factors were evaluated to determine associations with insurance provider including age, gender and race. Additional data obtained for each patient included geographic location of the hospital and risk factors for HCC defined as Hepatitis C, Hepatitis B, alcohol use disorder, non-alcohol fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis (now primary biliary cholangitis), autoimmune hepatitis, or other. Comorbidities were also evaluated using the Elixhauser comorbidity scale which was modified to exclude liver disease [9]. These variables were determined through the appropriate ICD-9 codes.
Statistical analysis: Associations between payer and each of the factors of interest were evaluated using chi square tests. Multivariable logistic regression models were fit for the presence of metastatic HCC, liver decompensation, and mortality and a multinomial logistic regression model was fit for treatment modality received where noninvasive treatment was the reference outcome. Terms in each of the models were determined through backward selection where hepatitis C, hepatitis B, alcohol, nonalcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis, autoimmune, liver decompensation, metastasis, Elixhauser comorbidity score, and treatment modality were eligible for inclusion, where appropriate. Further, age, gender, race, and geographic location of the hospital were fixed in all models. In all models, private insurance was treated as the reference payer category.
All analyses were performed using weighted data employing appropriate survey procedures to produce national estimates. Data was analyzed using SAS 9.4 (SAS Institute Inc. Cary, NC).
3Result3.1Patient demographicsThere was a total of 62,368 patients with HCC included in the study. The major identifiable insurance provider was Medicare, representing 44% (27,401) of the total patients, followed by private insurance (27%, 16,896), Medicaid (19%, 11,640), and other forms of payment (10%, 6431) (Table 1).
3.2Inpatient treatment for HCCThere were notable disparities in the treatment of HCC with significant differences based on the primary payer in the univariate analysis (p value <0.001). Patients with private insurance had higher rates of treatment compared to patients with Medicare, Medicaid, and other forms of payment. On multivariable analysis, patients with Medicare, Medicaid, and other forms of insurance were less likely to undergo liver transplant than patients with private insurance [(OR 0.63, 95% CI 0.47–0.84), (OR 0.23, 95% CI 0.15–0.33), (OR 0.26, 95% CI 0.15–0.45), respectively]. Patients with Medicare, Medicaid, and other forms of payment were also less likely to undergo resection than patients with private insurance [(OR 0.74, 95% CI 0.63–0.87), (OR 0.40, 95% CI 0.32–0.51), (OR 0.42, 95% 0.32–0.54), respectively]. Medicaid patients were less likely to undergo ablation as treatment for HCC compared to patients with private insurance (OR 0.52, 95% CI 0.40–0.68). Patients with other forms of insurance were less likely to undergo TACE compared to patients with private insurance (OR 0.64, 95% CI 0.43–0.96) (Table 2). These models were adjusted for age, gender, race, geographic region, hepatitis C, hepatitis B, alcohol, NASH, liver decompensation and Elixhauser comorbidity score.
3.3Liver severity, comorbidities, and risk for metastatic HCCOn univariate analysis, features of liver decompensation were significantly different between insurance providers (p value <0.001) (Table 1). Multivariable analysis demonstrated that patients with Medicaid, Medicare, or other forms of payment were more likely to present with liver decompensation than patients with private insurance [(OR 1.23, 95% CI 1.07–1.41), (OR 1.15, 95% CI 1.02–1.29), (OR 1.25, 95% CI 1.07–1.46), respectively] (Table 3) after adjusting for age, gender, race, geographic region, hepatitis C, alcohol, NASH, liver decompensation and Elixhauser comorbidity score.
| Intervention | Payer | Odds ratio | Confidence interval |
|---|---|---|---|
| Liver transplant | Private | Reference | |
| Medicare | 0.63 | 0.47, 0.84 | |
| Medicaid | 0.23 | 0.15, 0.33 | |
| Other | 0.26 | 0.15, 0.45 | |
| Resection | Private | Reference | |
| Medicare | 0.74 | 0.63, 0.87 | |
| Medicaid | 0.40 | 0.32, 0.51 | |
| Other | 0.42 | 0.32, 0.54 | |
| Ablation | Private | Reference | |
| Medicare | 1.17 | 0.93, 1.48 | |
| Medicaid | 0.52 | 0.40, 0.68 | |
| Other | 0.52 | 0.37, 0.74 | |
| TACE | Private | Reference | |
| Medicare | 0.99 | 0.81, 1.20 | |
| Medicaid | 0.76 | 0.53, 1.09 | |
| Other | 0.64 | 0.43, 0.96 | |
There was also a significant difference in the Elixhauser comorbidity score between payers (p value <0.001). Medicare patients were more likely to have a greater number of Elixhauser comorbidities with 59.37% of patients presenting with 3 or more comorbidities (Table 1).
The majority of patients presented with no evidence of metastasis, however there was a significant difference in the number of metastasized sites between payers on univariate analysis (p value 0.001) (Table 1). On multivariable analysis, patients with Medicaid were more likely to have metastatic HCC than patients with private insurance (OR 1.29, 95% CI 1.11–1.49) (Table 3) after adjusting for age, gender, race, geographic region, hepatitis C, alcohol, NASH, liver decompensation feature and Elixhauser comorbidity score.
3.4Inpatient mortalityInpatient mortality was significantly different between payers on univariate analysis (p value <0.001) (Table 1). After adjusting for confounders, patients with Medicare and Medicaid were less likely to have inpatient mortality compared to patients with private insurance [(OR 0.56, 95% CI 0.46–0.69), (OR 0.80, 95% CI 0.65–0.97), respectively] (Table 3) after adjusting for age, gender, race, geographic region, hepatitis C, hepatitis B, alcohol, NASH, liver decompensation, metastasis and treatment.
4DiscussionThis study shows that treatment disparities in patients with HCC continue to exist. The most notable of these are the disparities in the definitive curative therapies with liver transplantation and surgical resection based on insurance provider. However, disparities also exist in rates of ablation and TACE [7]. These disparities are likely influenced by higher rates of metastatic disease at time of diagnosis, decompensated liver disease, and other comorbidities in patients with government funded insurance, which have also been noted in previous studies [10–13]. These patients are less likely to undergo routine screening tests for liver cancer and have less opportunities to follow-up with a specialist. Despite recent changes in health care to promote treatment equity despite insurance provider, disparities in treatment continue to exist. It is crucial to recognize these disparities as treatment has a significant impact on patient outcomes and health care utilization in the United States.
Patients with Medicaid, Medicare, and other forms of non-private insurance are more likely to present with decompensated liver disease and other comorbidities than patients with private insurance. This is likely influenced by many factors, including availability to care and treatment, such as curative treatment for hepatitis C. Patients with Medicaid are also more likely to present with metastatic disease compared to patients with private insurance. Foremost, patients with these insurance providers are less likely to undergo routine screening exams for HCC. Previous studies have shown that only 38% of the eligible Medicaid patients underwent some form of imaging for HCC screening in a 15 month time frame [14]. Patients with Medicaid also have difficulty accessing providers, specifically specialists that are more likely to recommend screening exams for HCC. One study showed that only 21% of Medicaid patients with cirrhosis actually followed up with a gastroenterologist. When they followed up with a gastroenterologist they were more likely to undergo routine screening exams [14].
This study showed that patients with Medicaid, Medicare, and other forms of non-private insurance are less likely to undergo treatment for HCC than patients with private insurance. Part of this disparity may be related to increased rates of decompensated disease, metastatic disease, and other comorbidities in patients with government funded insurances which was seen in this study; however the cost of intervention likely factors into this disparity as well. It is suspected that the financial burden of HCC on the United States is greater than $450 million, which is an average of about $32,000 per patient considering that patients undergoing liver transplantation and surgical resection incur more costs than TACE and ablation [15]. This study shows that patients with private insurance were more likely to undergo the most expensive interventions, specifically transplant and resection compared to interventions like TACE and ablation which are significantly less expensive. This is further supported by patients with “other” insurance or payer which includes self-pay or charity cases being less likely to undergo any intervention for HCC. Given the financial burden of this disease, its complications, and impact on society, it is crucial to pick the best intervention at the lowest possible cost, recognizing that the best treatment for a specific patient may be more costly than other forms of treatment.
Patients with Medicaid, Medicare, and other forms of payment suffer worse clinical outcomes, specifically, quality of life and mortality than patients with private insurance. The 5-year survival rate of HCC is less than 20%; however, if patients undergo curative treatment, their disease-free survival and life expectancy exponentially increases. Several studies have shown that patients undergoing transplantation for HCC had a disease-free survival of greater than 75% at 5 years [16]. Patients with government funded insurance who receive less curative interventions would therefore be expected to have higher mortality rates overall. Interestingly, this study shows that Medicaid and Medicare patients actually have decreased mortality rates, however, it should be noted that this only reflects inpatient mortality. Patients with private insurance are more likely to undergo surgical intervention for HCC and therefore may have higher inpatient mortality rated as a result of complications. In addition, patients with government funded insurance may actually be pursuing comfort measures at home after hospital discharge.
Quality of life is also affected in patients that are less likely to undergo treatment. As this study shows in Table 1, these patients are more likely to present with features of decompensation such as ascites and hepatic encephalopathy. These complications may require frequent admissions and invasive therapies such as paracentesis [17].
Recognizing this disparity in healthcare is crucial in order to hypothesize ways to minimize or eliminate further inequality in healthcare. All patients with chronic liver disease should be referred to a hepatologist in order to facilitate care, however the patient's primary care physician should also promote screening exams for HCC in order to promote increased compliance with exams. Patient education and understanding of barriers to completing screening exams, such as transportation and cost should also be explored in patients with government funded insurances [18]. This could assist in earlier diagnosis when patients would be more likely to be a candidate for curative treatment. In addition, government funded insurance could consider linking reimbursement to completion of HCC screening as they are doing for other quality metrics in the care of diabetes and other malignancies.
There are multiple limitations in this study. First, the data was collected through the NIS where information can only be obtained from ICD-9 coding. These codes could not be verified by medical charts given privacy issues, and therefore are susceptible to error. It is expected that this error would be randomly spread out equally throughout the dataset and would not singularly impact a certain variable more than others. Given patient privacy, we were not able to perform a chart review on patients to obtain more information about the specific characteristics of a patient's tumor burden such as size number of lesions, or vascular invasion. These factors impact the choice of liver directed therapy, however, the extent of this effect could not determined in this study. The NIS does not include laboratory values, therefore we are unable to include the Model for End Organ Liver Disease (MELD) score or Child's Pugh Score, therefore disease severity was stratified by the number of features of decompensation. However, there are many strengths to this study including the large number of diverse patients with HCC. This number of patients could not be obtained with a single center study.
As the incidence and mortality of HCC continues to rise, it is vital to recognize the impact that treatment can have on patient mortality and quality of life. Given that liver transplantation and surgical resection are considered curative treatments for a disease with limited progress in survival rates thus far, it becomes even more important that all patients receive access to the best evidence-based treatment for their disease stage, regardless of their insurance status. Further research should be conducted to determine ways to reduce this disparity.AbbreviationsHCUP Healthcare Cost and Utilization Project hepatocellular carcinoma Institutional Review Board International Classification of Disease, Ninth Revision, Clinical Modification Nationwide Inpatient Sample transarterial chemoembolization
This research did not have grant or other financial support.
Conflict of interestThe authors have no conflicts of interest to declare.