Certain neuroendocrine tumors (NET) metastasized to the liver can resemble hepatocellular carcinoma (HCC) in cytological needle biopsy. Experience concerning the histologic characteristics of metastatic NET resembling HCC in core needle hepatic biopsies has been scarce. The aim of this study is to describe the histological criteria in seven metastatic NET that resembled HCC in core needle hepatic biopsy. From a total of 285 needle biopsies with primary or metastasized hepatic neoplasms, seven cases were selected originally diagnosed as HCC or HCC vs. NET metastasized to the liver. Fourteen needle biopsies of hepatocellular carcinomas were also studied for comparative purposes. In all of these neoplasms the diagnosis of endocrine tumor was confirmed by immunohistochemical studies and the following information was recorded: age, sex, radiological alterations, primary site of the NET, and follow-up. The following histological data were also recorded: fibrotic stroma associated or not with the neoplastic cells, growth pattern, form of the cells, cellular size, mitotic figures, nucleomegaly, apparent nucleoli, chromatin in salt and pepper, plasmacytoid cells, intranuclear inclusions, and biliary pigment.
In conclusion, these characteristics were common in metastasized neuroendocrine tumors: extensive stro-mal fibrosis, slight to moderate atypia, hyperchromatic nuclei, plasmacytoid cells, and thin delicate strands of fibrovascular tissue supporting larger acinar groups of net cells. HCC had a more infrequent fibrotic stroma, moderate to marked atypia, and in some biopsies biliary pigment, intranuclear inclusions, and clear cells.
Neuroendocrine tumors (NET) comprise a wide group of neoplasms including carcinoids in diverse locations, tumors of the pancreatic islets, small cell tumors, medullary carcinomas of the thyroid, and others.1 Although all of these neoplasms produce neuroendocrine granules, their histological aspect varies depending on their degree of differentiation. Well differentiated NET is common in the gastrointestinal tract and in the pancreas, and present nests of cells surrounded by fibrovascular stroma arranged in a nodular or trabecular growth pattern (organoid pattern). Poorly differentiated NET may or may not have this organoid pattern and include small cell lung carcinoma and large-cell neuroendo-crine carcinoma.1-2 As a group, malignant NET are larger in size, have a higher mitotic index, necrosis, and vascular invasion. Intestinal and pulmonary tumors with an intermediate degree of differentiation have been denominated as atypical carcinoids.2
The liver is a common site for metastasis of endocrine neoplasms originating in the gastrointestinal tract, pancreas, biliary system, or lungs.3-5 Sometimes the metastasis is the first or only evidence of a malignant NET, and the site of the primary tumor may be located months or years later, since primary hepatic carcinoids are very rare.6
Neuroendocrine tumors metastasized to the liver have mainly been studied in percutaneous fine-needle aspiration biopsies. Several reviews have recognized that these tumors may resemble hepatocellular carcinoma (HCC) or adenocarcinomas.4,5,7 In contrast, experience with metastatic NET in core needle biopsies is limited.4-5 The purpose of this review is to describe the morphology and immunohistoche-mical pattern for seven cases of metastatic NET that resembled hepatocellular carcinomas in core needle biopsies of the liver.
Material and MethodsOut of a total of 285 hepatic biopsies with primary and metastatic hepatic tumors obtained. We selected seven cases of NET that were diagnosed originally as HCC or that suggested a differential diagnosis of HCC us. metastatic NET. In five of these seven cases, neu-roendocrine neoplasms were found in the pancreas or ileum after the hepatic biopsy supporting a diagnosis of endocrine neoplasms metastasized into the liver (Table 1). The diagnosis of endocrine tumor in these cases was based on immunohistochemical studies (chromogranin and synaptophysin). The other two cases were recognized as endocrine neoplasms during the retrospective review because they had isolated histolo-gical fields with either trabecular areas or organoid pattern, suggesting neuroendocrine tumors. Both cases were positive for Chromogranin and Synaptophy-sin and negative for Hepar-l. Hepatic tumors with the characteristic aspect of metastasized neuroendocrine tumors were excluded from the review since they were not a diagnostic problem.
| Patients | Age and Sex | Admitting Diagnosis | Size and number of hepatic lesions | Location | Follow-up | Original diagnosis in Biopsy (H&E) | Primary neoplasm m |
|---|---|---|---|---|---|---|---|
| 1 | 80 years male | Hepatic tumor | Single 20 cm | Both lobes | Alive/6 months | HCC vs. endocrine tumor | Pancreas |
| 2 | 56 years Female | Hepatic and | Multiple, 1 3 cm. pancreatic tumor | Right lobe | Alive/1 year | HCC | Pancreas |
| 3 | 52 years Female | Probable HCC | Single I2cm | Right lobe | Dead/3 years | HCC vs. endocrine tumor | Ileum |
| 4 | 48 years Male | Hepatic tumor | Single 8 cm. | Right lobe | Alive/3 years years | HCC | Ileum |
| 5 | 22 years Female | Probable HCC | Single 24 cm | Both lobes | Alive/6 months | HCC | Unknown |
| 6 | Hepatic tumor | Single 18 cm. | Both lobes | Unknown | HCC vs. endocrine tumor | Unknown | |
| 7 | 50 years Male | Hepatic tumor | Single 14 cm | Right lobe | Alive 2/years | HCC | Pancreas |
HCC: Hepatocellular carcinoma.
The following clinical data were noted: age, sex, diagnosis at admission, radiological data, primary site of the NET, and follow-up. In all cases the slides were stained with H8E and Masson and the following data were recorded: fibrotic stroma associated or not with neoplastic cells, growth pattern, cell shape, cell size, mitotic figures, aspect of the nucleus and nucleolus, chromatin in salt and pepper, aspect of the cytoplasm, plasmacytoid cells, sinusoids between neoplastic cells, intranuclear inclusions, and biliary pigment. For all seven cases immunohistochemical studies with the following antibodies were performed: Chromogranin (1:200), Dako Carpentaria, CA; Synaptophysin (1:200), Bio-care Medical Concord, CA and Hepar-1, (1:500). The neoplasm was considered to be neuroendocrine when Synaptophysin and/or Chromogranin were positive and Hepar-1 was negative.
For comparative purposes we studied 14 cases of HCC. For all of them we recorded the histological criteria mentioned above, and also noted the presence of portal fibrosis and clear cells due to deposits of fat or glycogen. These neoplasms were positive to Hepar-1 and negative for Chromogranin and Sy-naptophysin.
Core needle biopsy was performed under radiolo-gic guidance, using ultrasound or computerized tomography with an 18-auge Biopsy (Medi Tech Corp.).
ResultsClinical findingsThe principal clinical and radiological findings for the neuroendocrine tumors are shown in table 1. Age varied between 22 and 80 years old (with an average of 50 years old). Admitting diagnosis was hepatic tumor or probable HCC. During the study of the patient the primary site was found to be the pancreas in three patients. In another two of them, the neoplasm was located in the ileum one month and two years after the hepatic neoplasm was diagnosed, and in another two cases the primary site had not been found 4 and 6 months after hepatic neuroendo-crine tumor had been diagnosed. In radiological studies the neoplasm was found on the right hepatic lobe in 4 patients and in both lobes in the other three patients. No patient had carcinoid syndrome. Five patients were still alive several months or years after the metastatic NET had been diagnosed, one died, and the follow-up of the remaining one was unknown.
Histological findings (Tables 2 and 3)- •
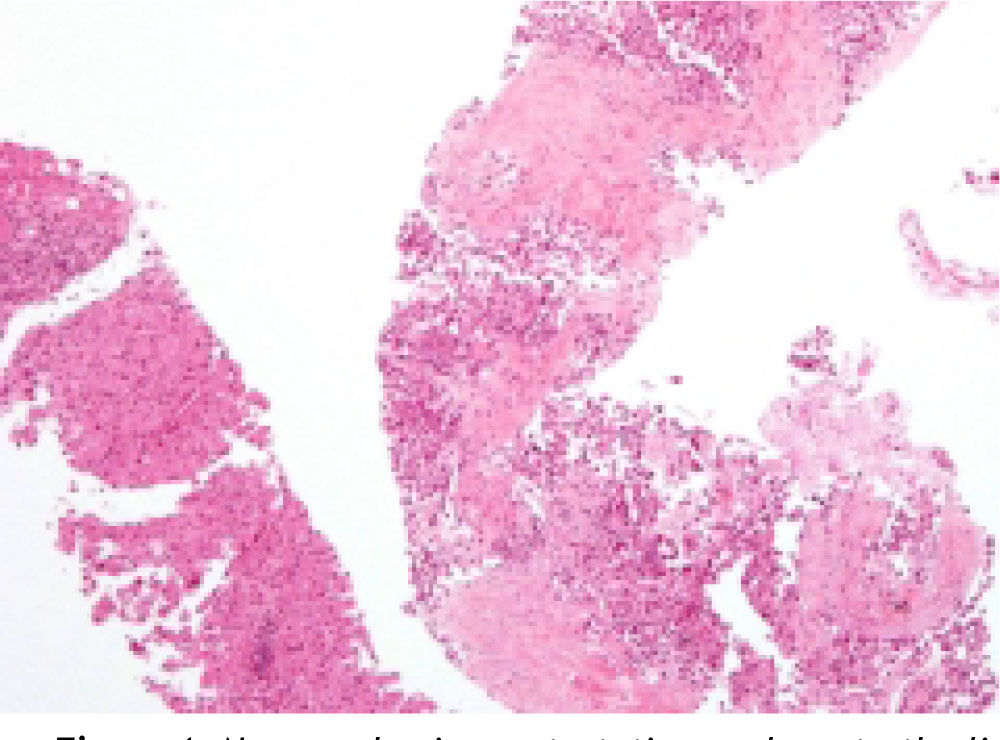
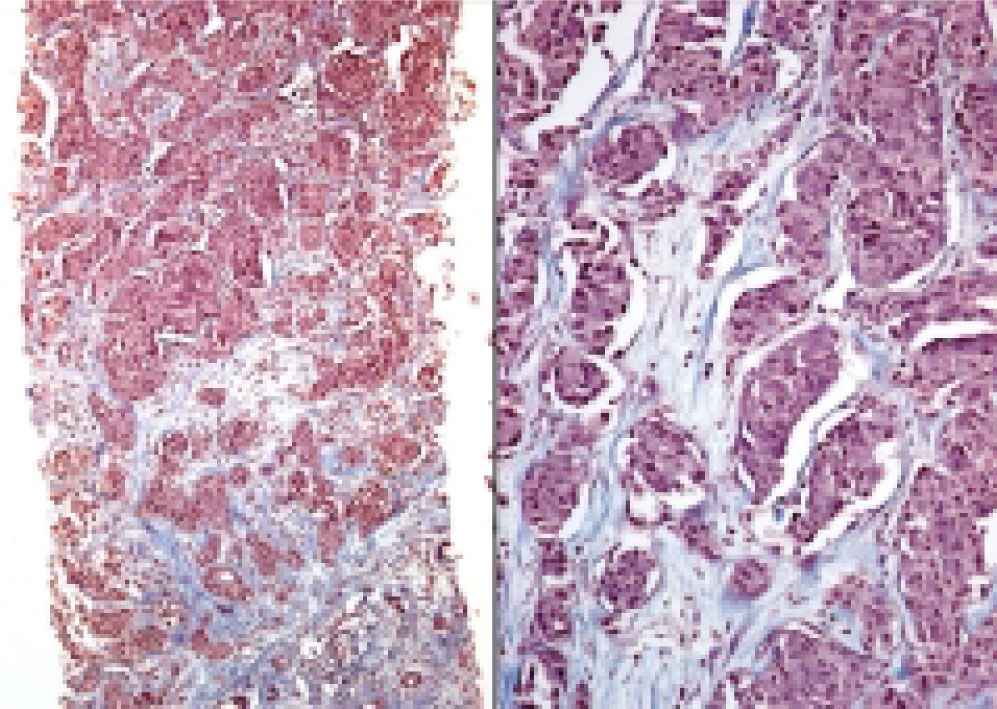
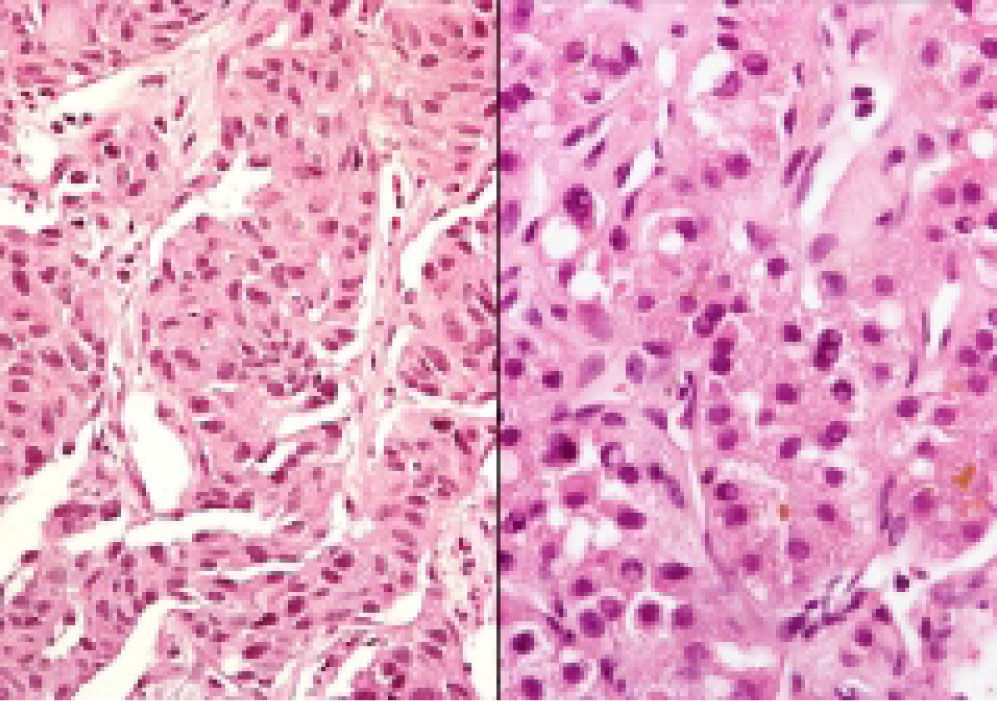
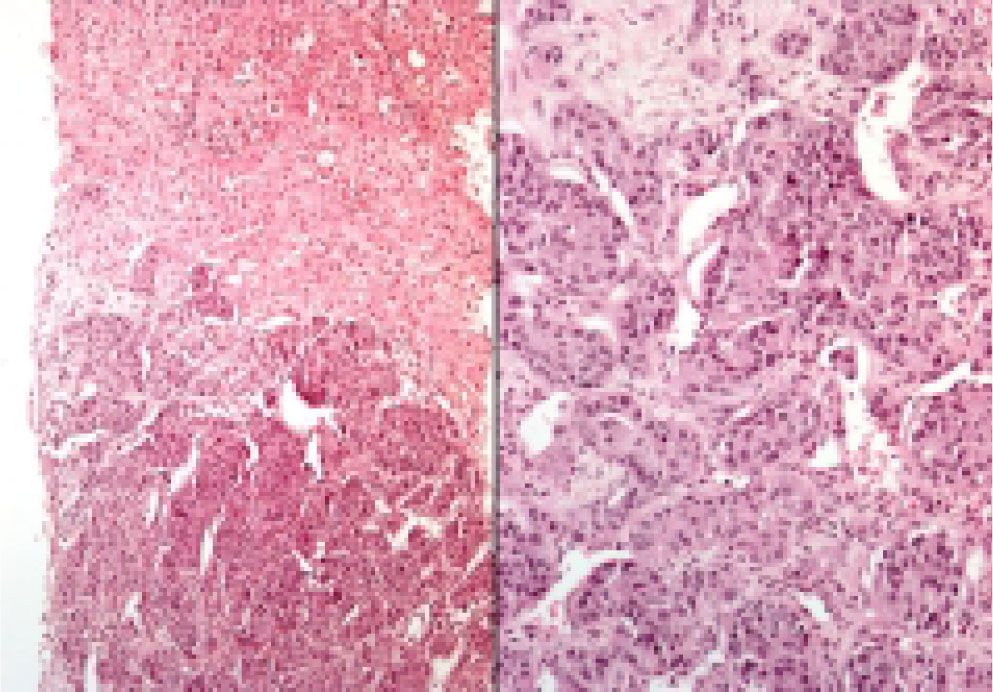
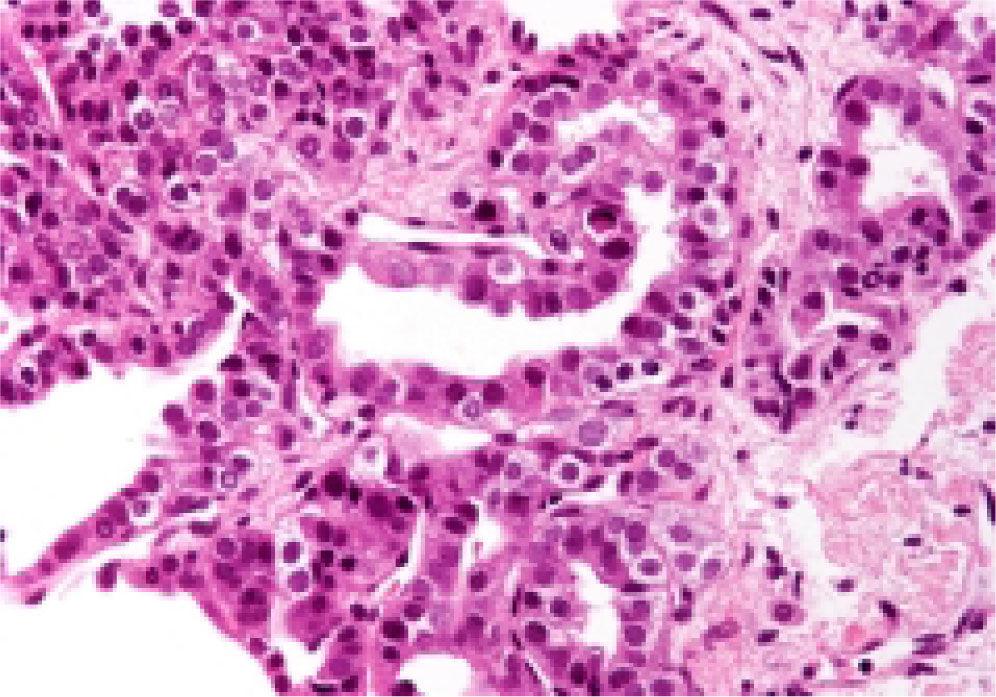
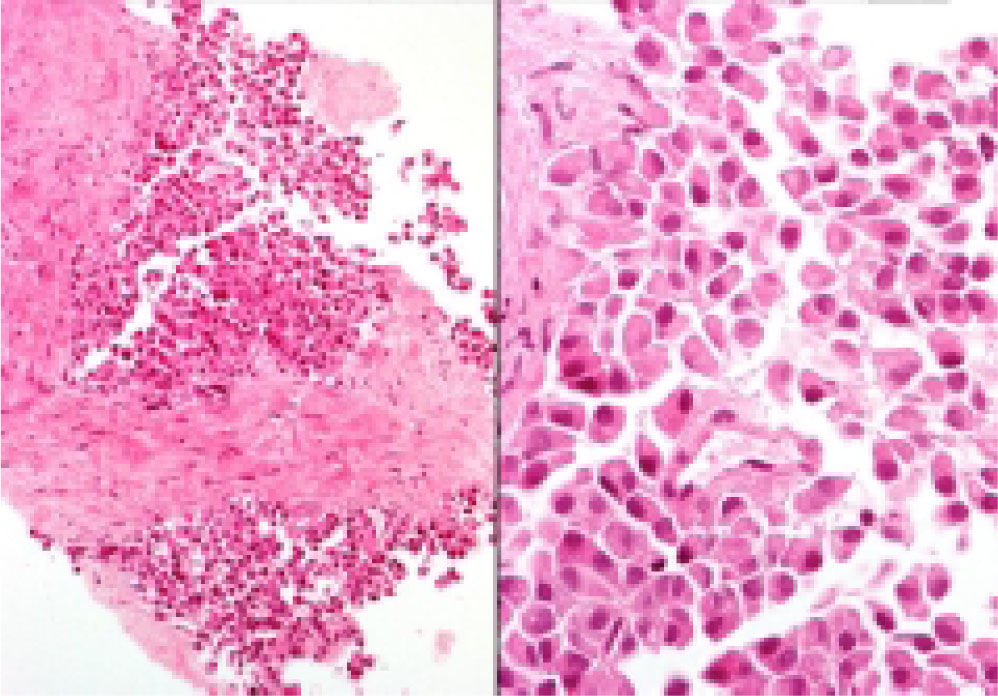
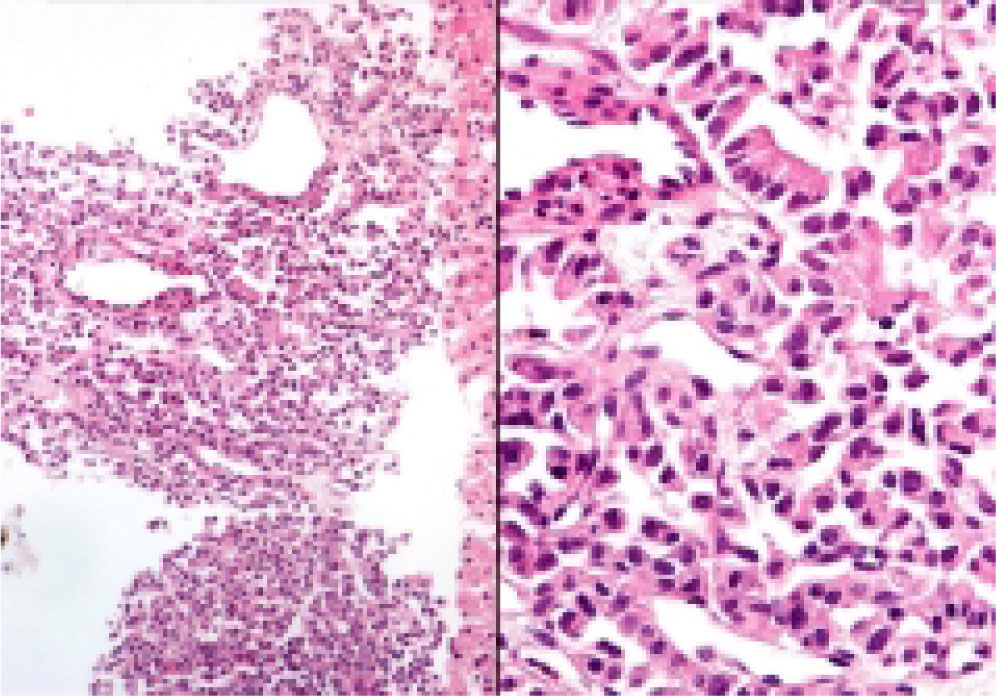
Neuroendocrine neoplasms: The seven biopsies with metastatic NET showed fibrotic stroma in varying degrees (Figure 1). In some fields the fibrosis surrounded the neoplastic cells and two cases had areas similar to the ones observed in fi-brolamellar HCC (Figure 2). The most frequent growth pattern was trabecular, which in several cases resembled the trabecular pattern of HCC (Figure 3). In other areas the NET had cellular nests with well defined limits (Figure 4) or with pseudoglandular features (Figure 5). In five cases there were round or ovoid cells of medium size with a monotonous appearance, and in the other two there were larger and moderately atypical cells. Most of the nuclei were either round or ovoid with hyperchromatic features. In none of the cases did we observe distribution in salt-and-pepper chromatin. Nucleoli were either absent or una-pparent in two cases. Cells with a plasmacytoid aspect were found in 6 of 7 biopsies in varying quantities (Figure 6). In six of the seven cases the neoplastic cells were separated by sinusoids coated by endothelial cells and in some fields had thin, delicate strands of fibrovascular tissue supporting groups of neuroendocrine cells (Figure 7).
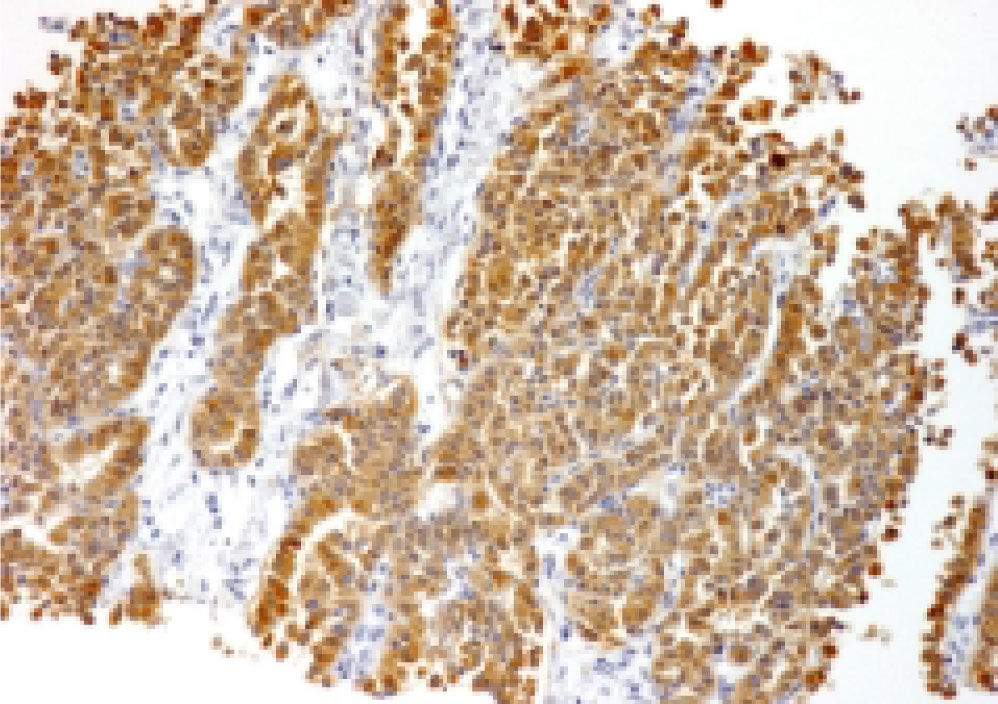
Intranuclear inclusions were scarce and found in two cases. In none of the biopsies did we find bile or other cytoplasmic pigments. Mitosis were either absent or seen in isolated fields. These neoplasms were positive to chromogranin and synaptophisin (Figure 8) and negative for HE-PAR-I.
- •
Hepatocellular carcinomas: In addition to areas with portal fibrosis or cirrhosis (7 biopsies), the HCC also had areas with a fibrotic stroma which in certain sites surrounded nests of neoplastic hepatocytes. The cells were ovoid and spindle of medium size and in some sites alternated with polygonal cells of large size. Nuclear atypia was moderate to marked, and as a group the cells of HCC included pleomorphic cells of a very large size with nucleomegaly, apparent nucleoli, and occasional mitosis. The neoplastic cells were separated by sinusoids (Figure 3) but in none of the cases did the neoplastic cells coat a delicate fibrovascular stroma as observed in the neuroendocrine neoplasms (Figure 7). Intranuclear inclusions and bile pigment (Figure 3, right) were found in 8 of the 14 HCC. In none of the cases of HCC did we identify cells with a plasma-cytoid appearance.
Histological findings in core neeedle hepatic biopsy
| MNET (7 TUMORS) | HCC (14 TUMORS) | |
|---|---|---|
| Fibrotic Stroma | 7 | 6 |
| Ovoid cells | 5 | 6 |
| Ovoid and spindle cells | 2 | 4 |
| Ovoid and polygonal cells | 0 | 4 |
| Nucleomegaly | 2 | 11 |
| Hyperchromatic nuclei | 6 | 5 |
| Plasmacytoid cells | 6 | 0 |
| Sinusoids | 6 | 12 |
| Intranuclear inclusions | 2 | 8 |
| Nucleoli | 2 | 12 |
| Biliary pigment | 0 | 8 |
| Salt-and-pepper chromatin | 0 | 0 |
| Clear cells | 0 | 3 |
MNET: Metastatic neuroendocrine tumors. HCC: Hepatocellular carcinomas.
Histological features in core needle hepatic biopsy.
| MNET | HCC | |
|---|---|---|
| Fibrotic stroma | Moderate to marked | Moderate |
| Cell size | Small/moderate | Moderate/large |
| Nucleomegaly | Occasional | Common |
| Hyperchromatic nuclei | Common | Occasional |
| Plasmacytoid cells | Common | Absent |
| Sinusoids in the neoplasm | Common | Common |
| Intranuclear inclusions | Rare | Common |
| Clear cells | Absent | Occasional |
MNET: Metastatic neuroendocrine tumors. HCC: Hepatocellular carcinomas.
The diagnosis of metastatic NET is relatively easy to establish since most of them keep the architectural and cytological aspect of the primary neuroendocrine neoplasms. However, some neuroendocrine metasta-tic tumors may have a cytological and architectural aspect similar to HCC.4-6,8-9 Most metastatic NET in fine needle aspiration biopsies show typical endocrine features, however some of them may display cytologi-cal characteristics such as increased cytoplasm, nu-cleoli and a lack of salt and pepper chromatin which may cause confusion with HCC.4,5,7,10
The literature on core needle biopsy of metastatic NET that exhibits a hepatoid appearance is limited. In the cytohistological series by Prosser et al.,4 4 of 10 core needle hepatic biopsies with neuroendocrine neoplasms were originally interpreted as HCC. These authors found that the core needle biopsies of these neoplasms show two patterns of architecture. In one, the main feature was an abundant fibrotic stroma associated with thin narrow trabecules of tumor cells. The other pattern showed thin, delicate strands of fibrovascular tissue supporting larger aci-nar groups of net cells. While the nuclear features were indistinct on the H 8 E stained section, the plasmacytoid shape of these cells were prominent and the cells retained their round or oval shape.
We found that the fibrotic stroma is very useful in suspecting a neuroendocrine metastatic tumor, but a fibrotic stroma with a similar aspect may also be observed in biopsies of HCC. In both cases the fibro-sis may surround nests or trabecules of neoplastic cells and show a desmoplastic pattern. The confusion may be greater in biopsies with neuroendocrine carcinomas in which there is interstitial fibrosis that may resemble fibrolamellar carcinomas.11 The growth pattern in neuroendocrine neoplasms and HCC can also be similar and both may have ovoid or spindle cells arranged in a trabecular, nodular or pseudoglandular pattern divided by sinusoids.12
The most notable differences between both neoplasms in this series included major nuclear atypia, nucleomegaly, prominent nucleoli, biliary pigment, intranuclear pseudoinclusions, and clear cells in biopsies with HCC. Although intranuclear pseudoin-clusions are present most frequently in HCC they may also be observed isolated in metastatic neuroen-docrine neoplasms.5 In this series two of the seven biopsies had pseudoinclusions in isolated histologi-cal fields. On the other hand it must be emphasized that the salt-and-pepper chromatin commonly observed in the cytological study4,5 was rare in needle biopsies of the liver and was found in none of the seven cases we studied here.
Although some biopsies of HCC may present a cytological aspect very similar to the one in endocrine neoplasms, HCC do not exhibit cells with a plasma-cytoid appearance. In contrast, plasmacytoid cells were present in 6 of the 7 endocrine neoplasms in varying quantities and were found arranged in irregular nests surrounded by fibrotic stroma or coating thin and delicate strands of fibrovascular tissue.
A diagnosis of neuroendocrine neoplasm metas-tasized into the liver must be suspected in a needle biopsy when plasmacytoid cells are found intermingled with broad bands of connective tissue or associated with delicate strands of fibrovascular tissue. However, in some biopsies the plasmacytoid cells are very scarce and the neoplasm has a trabecular or nodular growth pattern and cells with slight to moderate atypia. If in doubt, the distinction between these neoplasms must be established with immunohistochemical stains. Positivity to Synapto-physin and/or Chromogranin and negativity to He-par-1 are virtually diagnostic of metastatic NET. Like other authors, in our experience synaptophy-sin is the most useful neuroendocrine marker in biopsies with neuroendocrine tumors metastasized to the liver.4
Although the majority of malignant neuroendo-crine neoplasms are metastatic it should be noted that there have been reports of primary HCC with a neuroendocrine component.13 In two of the seven cases in this series no primary neoplasm had been identified several months after the neuroendocrine tumor had been diagnosed, and only the evolution and/or additional studies would make it possible to classify them with confidence as primary or metasta-tic neoplasms.
The distinction between HCC and metastatic neu-roendocrine tumors is crucial to their prognosis and treatment. Surgical excision of neuroendocrine me-tastatic neoplasms has a better prognosis than HCC14,15 and prolonged life expectancies can be obtained. Most HCC, on the other hand, are only curable when they are small and the diagnosis is established early.16-17
AcknowledgementsThe authors are grateful to Dr. Sharon Ortiz Arce (Master in Medical Sciences), for her valuable help in the fulfillment of the immunohistochemical studies.