Introduction and aim. This manuscript seeks to analyze the impact of lifestyle changes on body mass index (BMI), aminotransferases and steatosis in children and adolescents with nonalcoholic fatty liver disease (NAFLD).
Material and methods. A review of PubMed, BIREME, Scopus, EMBASE, Medline and Web of Science databases 2015 was performed seeking studies addressing the impact of lifestyle interventions on children and/or adolescents with NAFLD. Inclusion were manuscripts written in Portuguese, English and Spanish, as well as age less than 18 years. Two reviewers performed the data extraction independently and differences were resolved by consensus. Outcome measures were BMI, serum aminotransferase levels and the presence of hepatic steatosis.
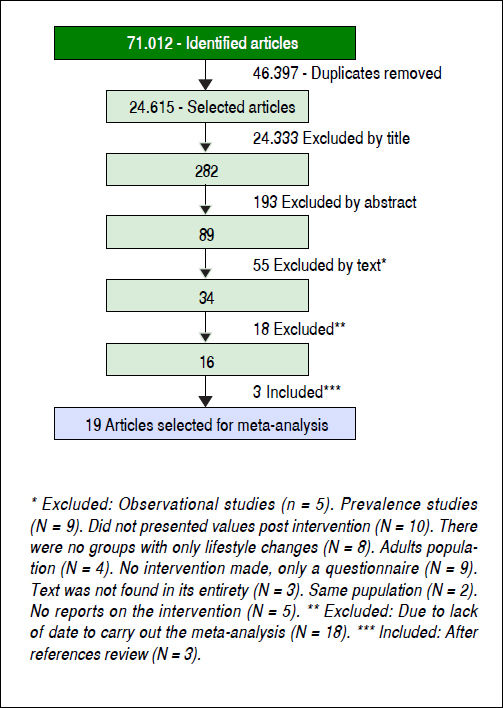
Results. The literature search identified 71,012 articles. After excluding 46,397 duplicates and other clearly irrelevant studies, 89 publications were reviewed in detail. Another 55 studies were excluded at this stage. Subsequently, 18 were excluded due to lack of data and three new articles were found in the review of the references of previously identified manuscripts. Therefore, 19 studies that had evaluated 923 subjects (477 boys and 446 girls) aged 6-18 years were included in the review. In most studies, the intervention included aerobic exercise and diet. In nine studies, BMI improved significantly following the intervention. The vast majority of studies reported a benefit from the intervention on aminotransferase levels. Lifestyle changes also had a significant impact on steatosis, reducing the risk by 61%.
Conclusion. In conclusion lifestyle changes lead to significant improvements in BMI, aminotransferase levels and hepatic steatosis in children and adolescents with NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is an increasingly common cause of chronic liver disease in children and adolescentes,1 likely due to an increase in the incidence of overweight and obesity in this population.2,3 In Europe, the prevalence of overweight and obesity in children surpasses 30% and has been steadily increasing over the past decades.3 In the United States, 16.9% of children aged 2 to 19 are obese.4 In Brazil, more than 30% of children between the ages of 5 and 9 are overweight, while the prevalence of obesity is about 15%.5
NAFLD is classified histologically as either simple hepatic steatosis or non - alcoholic steatohepatitis (NASH), the latter being defined by hepatocellular steatosis and inflammation with or without fibrosis.6 The gold standard for the diagnosis of NAFLD is histology obtained using liver biopsy, which can determine the presence of steatosis, inflammation or fibrosis.2 The biopsy has some limitations; therefore, indirect methods have been used for diagnosis.7–10
NASH patients are more likely to develop progressive liver disease, such as cirrhosis and hepatocellular carcinoma, and have increased risk of developing cardiovascular events.11–14 The long-term prognosis of children with NAFLD remains nuclear;12 however, in adulthood these patients have a 14-fold increased risk of dying or requiring liver transplantation compared to the general population of the same age and sex.15
Due to the correlation of NAFLD with excess weight, the first step in treatment has traditionally been targeting obesity by stimulating lifestyle changes through physical activity and consumption of a healthier diet, as this is the only available treatment for these patients.16–18 The number of well-designed, randomized controlled trials that have investigated the impact of lifestyle changes as a first-step intervention to treat pediatric NAFLD is small. Most intervention studies that combine diet and increased physical activity shows a benefit in NAFLD. Exercise, even without weight loss, appears to reduce steatosis, but the intensity of the exercise for the treatment of NAFLD has not yet been established. Isolated exercise, without caloric restriction, is not yet a proven strategy for the management of children with NAFLD.19–23
The aim of this study was to perform a systematic review and meta-analysis to assess the evidence on the impact of lifestyle interventions through aerobic exercise and a balanced diet on body mass index (BMI), serum aminotransferase levels and hepatic steatosis in children and adolescents NAFLD.
Material and MethodsType of studies assessedThe search included randomized controlled trials, cohort, case-control studies, non-randomized controlled trials and non-controlled trials. Case reports, reviews, editorials and thesis dissertations were excluded. We included manuscripts published in Portuguese, English or Spanish.
Types of exposure and participantsThe trials included had investigated some form of lifestyle intervention, such as regular physical activity, diet or a combination of the two. Randomized trials that had investigated medications for the treatment of NAFLD were included but only data from the control arm were included in the analyses. Studies were included if variables BMI, aminotransferases and hepatic steatosis were available before and after the intervention.
Studies including participants under 18 years of age were included. In cases of combined adult and pediatric cohorts, data from individuals under the age of 18 years were included. All participants had to have a diagnosis of NAFLD for inclusion in the analysis. The diagnosis could have been made clinically (overweight/obesity patient with abnormal aminotransferases or increased insulin resistance), radiologically (magnetic resonance imaging of the abdominal (MRI)), ultrasound or histologically following a liver biopsy.24,25
We excluded studies that evaluated children and adolescents with other chronic liver diseases such as infectious hepatitis, endocrinological diseases that could predispose to liver disease, steatosis secondary to parenteral nutrition, drugs or alcohol and studies that had evaluated the patients after surgery.
Types of outcome measuresStudies were included if the outcome measures of interest were improvement in BMI, serum aminotransferase levels, as well as radiological or histological evidence of steatosis improvement. To compare BMI between ages and gender Z scores were used. A BMI between 85 and 96 percentiles for age was used to define overweight and percentile ≥ 97 was used as cut off for obesity.1,26 Aminotransferases (ALT, AST) were used as the only laboratory test; the values were defined according to the laboratory reference of each study.16
Search methods and selection of studiesPubMed, BIREME, Scopus EMBASE, Medline and Web of Science were reviewed by a single researcher. The search in the database covered articles published throughout the indexing period up to April 2015.
The literature search used the following key words: disease (hepatic steatosis non-alcoholic, non-alcoholic steatohepatitis, NAFLD, NASH, obesity, aminotransferase, aspartate aminotransferase AST, ALT, alanine aminotransferase, fatty liver and hepatic steatosis). These words were combined with the use of the following words: lifestyle intervention, physical activity, physical fitness, aerobic exercise, weight loss, intervention, treatment, nutritional intervention and diet.
Two reviewers (MUM and CTF) performed the data extraction independently and differences were resolved by consensus. Outcome measures were: BMI, serum levels of ALT and AST and the presence of hepatic steatosis, before and after the intervention. In each study data on participant’s characteristics, such as gender, age and the way in which the intervention was performed were extracted. In cases of missing data, the corresponding author was contacted via email twice.
The effects of quantitative parameters were estimated using the patterned effect. For categorical parameters, we used the relative risk. For both effective measures, we calculated intervals of 95% confidence. Q test was used to assess for heterogeneity of the studies and in the case of heterogeneity being greater than expected, we used the random effects model to obtain aggregated measurements. The significance level was 5% and the analysis were performed in STATA version 13.0.
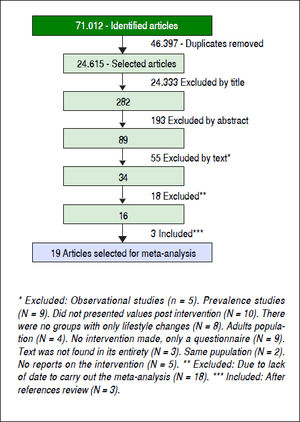
ResultsFollowing the initial search 71,012 articles were identified (Figure 1). After excluding 46,397 duplicates, 24,615 articles were reviewed and 24,333 were excluded. The abstracts of the 282 remaining manuscripts were reviewed, which led to the removal of another 193 studies. Eighty-nine manuscripts were read in their entirety leading to the exclusion of another 55 studies. Of the remaining 34 manuscripts 18 were not included, as they did not contain data necessary for the meta-analysis. Three more studies identified by reviewing the references of already included studies were selected as well. In total, 19 manuscripts (923 individuals) were selected for review and meta-analysis.
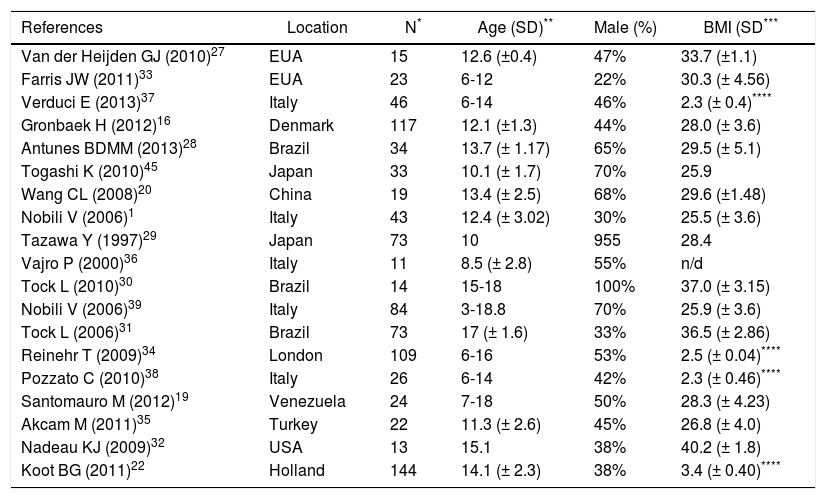
Out of the selected studies eight were controlled trials, three were nonrandomized and eight were non-controlled clinical trials. In most studies, the intervention included aerobic exercise and diet. In two studies27,28 physical activity through aerobic exercise was the only intervention. The age of participants ranged from 6 to 18 years, and 477 were male and 446 females (Table 1).
Characteristics of the evaluated population.
| References | Location | N* | Age (SD)** | Male (%) | BMI (SD*** |
|---|---|---|---|---|---|
| Van der Heijden GJ (2010)27 | EUA | 15 | 12.6 (±0.4) | 47% | 33.7 (±1.1) |
| Farris JW (2011)33 | EUA | 23 | 6-12 | 22% | 30.3 (± 4.56) |
| Verduci E (2013)37 | Italy | 46 | 6-14 | 46% | 2.3 (± 0.4)**** |
| Gronbaek H (2012)16 | Denmark | 117 | 12.1 (±1.3) | 44% | 28.0 (± 3.6) |
| Antunes BDMM (2013)28 | Brazil | 34 | 13.7 (± 1.17) | 65% | 29.5 (± 5.1) |
| Togashi K (2010)45 | Japan | 33 | 10.1 (± 1.7) | 70% | 25.9 |
| Wang CL (2008)20 | China | 19 | 13.4 (± 2.5) | 68% | 29.6 (±1.48) |
| Nobili V (2006)1 | Italy | 43 | 12.4 (± 3.02) | 30% | 25.5 (± 3.6) |
| Tazawa Y (1997)29 | Japan | 73 | 10 | 955 | 28.4 |
| Vajro P (2000)36 | Italy | 11 | 8.5 (± 2.8) | 55% | n/d |
| Tock L (2010)30 | Brazil | 14 | 15-18 | 100% | 37.0 (± 3.15) |
| Nobili V (2006)39 | Italy | 84 | 3-18.8 | 70% | 25.9 (± 3.6) |
| Tock L (2006)31 | Brazil | 73 | 17 (± 1.6) | 33% | 36.5 (± 2.86) |
| Reinehr T (2009)34 | London | 109 | 6-16 | 53% | 2.5 (± 0.04)**** |
| Pozzato C (2010)38 | Italy | 26 | 6-14 | 42% | 2.3 (± 0.46)**** |
| Santomauro M (2012)19 | Venezuela | 24 | 7-18 | 50% | 28.3 (± 4.23) |
| Akcam M (2011)35 | Turkey | 22 | 11.3 (± 2.6) | 45% | 26.8 (± 4.0) |
| Nadeau KJ (2009)32 | USA | 13 | 15.1 | 38% | 40.2 (± 1.8) |
| Koot BG (2011)22 | Holland | 144 | 14.1 (± 2.3) | 38% | 3.4 (± 0.40)**** |
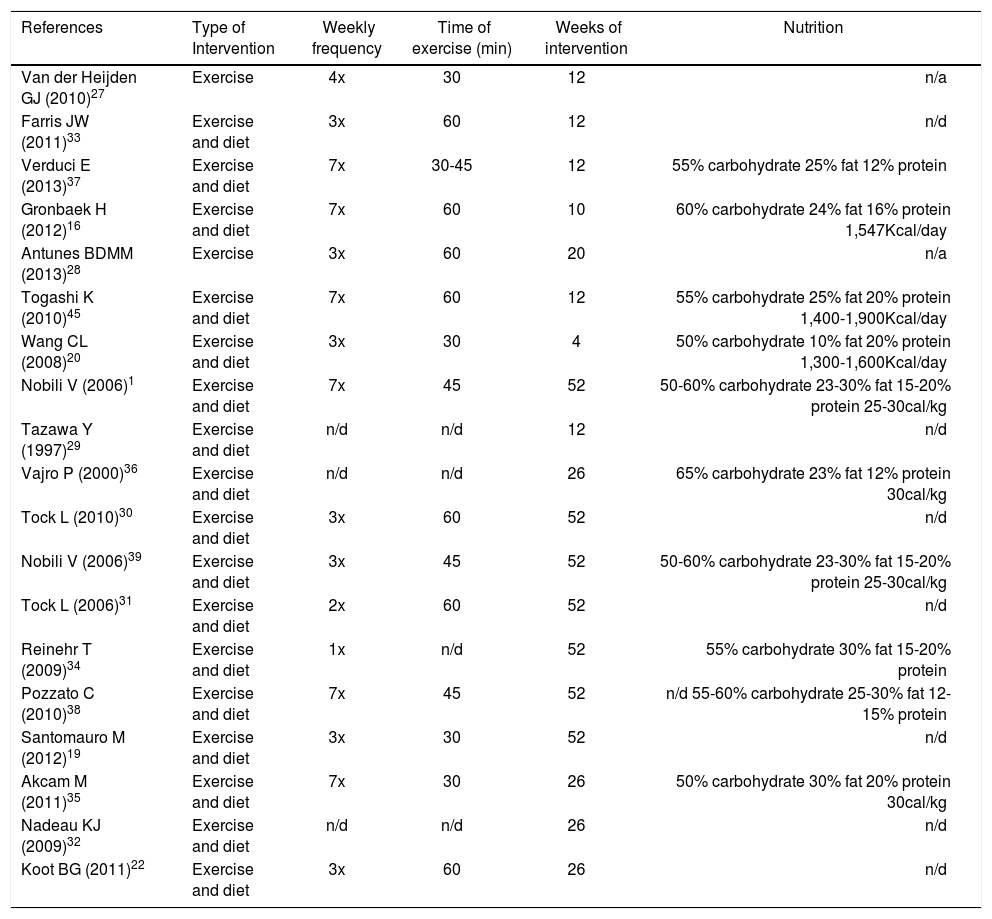
The diet suggested was normocaloric ranging from 1.300-1900 Kcal/day with a caloric distribution as follows: 50-65% from carbohydrates, 10-30% from fat and 12-20% from protein. In some studies, depending on the patient’s age, 10 g of fiber were added to the diet. The details of the diet were not reported in seven studies.19,22,29–33 Regarding physical activity, all studies assessed aerobic exercise, ranging from once a week to daily workouts of 60 minutes on average. The duration of intervention ranged between 4 and 52 weeks. Details of interventions are shown in table 2.
Details of the interventions used in each study for lifestyle change.
| References | Type of Intervention | Weekly frequency | Time of exercise (min) | Weeks of intervention | Nutrition |
|---|---|---|---|---|---|
| Van der Heijden GJ (2010)27 | Exercise | 4x | 30 | 12 | n/a |
| Farris JW (2011)33 | Exercise and diet | 3x | 60 | 12 | n/d |
| Verduci E (2013)37 | Exercise and diet | 7x | 30-45 | 12 | 55% carbohydrate 25% fat 12% protein |
| Gronbaek H (2012)16 | Exercise and diet | 7x | 60 | 10 | 60% carbohydrate 24% fat 16% protein 1,547Kcal/day |
| Antunes BDMM (2013)28 | Exercise | 3x | 60 | 20 | n/a |
| Togashi K (2010)45 | Exercise and diet | 7x | 60 | 12 | 55% carbohydrate 25% fat 20% protein 1,400-1,900Kcal/day |
| Wang CL (2008)20 | Exercise and diet | 3x | 30 | 4 | 50% carbohydrate 10% fat 20% protein 1,300-1,600Kcal/day |
| Nobili V (2006)1 | Exercise and diet | 7x | 45 | 52 | 50-60% carbohydrate 23-30% fat 15-20% protein 25-30cal/kg |
| Tazawa Y (1997)29 | Exercise and diet | n/d | n/d | 12 | n/d |
| Vajro P (2000)36 | Exercise and diet | n/d | n/d | 26 | 65% carbohydrate 23% fat 12% protein 30cal/kg |
| Tock L (2010)30 | Exercise and diet | 3x | 60 | 52 | n/d |
| Nobili V (2006)39 | Exercise and diet | 3x | 45 | 52 | 50-60% carbohydrate 23-30% fat 15-20% protein 25-30cal/kg |
| Tock L (2006)31 | Exercise and diet | 2x | 60 | 52 | n/d |
| Reinehr T (2009)34 | Exercise and diet | 1x | n/d | 52 | 55% carbohydrate 30% fat 15-20% protein |
| Pozzato C (2010)38 | Exercise and diet | 7x | 45 | 52 | n/d 55-60% carbohydrate 25-30% fat 12-15% protein |
| Santomauro M (2012)19 | Exercise and diet | 3x | 30 | 52 | n/d |
| Akcam M (2011)35 | Exercise and diet | 7x | 30 | 26 | 50% carbohydrate 30% fat 20% protein 30cal/kg |
| Nadeau KJ (2009)32 | Exercise and diet | n/d | n/d | 26 | n/d |
| Koot BG (2011)22 | Exercise and diet | 3x | 60 | 26 | n/d |
n/a: not evaluated n/d. n/d: unavailable.
In most studies, hepatic steatosis was assessed using abdominal ultrasound.16,19,20,22,28–32,34–36 Three studies assessed steatosis through MRI/MR spectroscopy27,37,38 and only two studies included histological data as a means of assessing steatosis severity.1,39
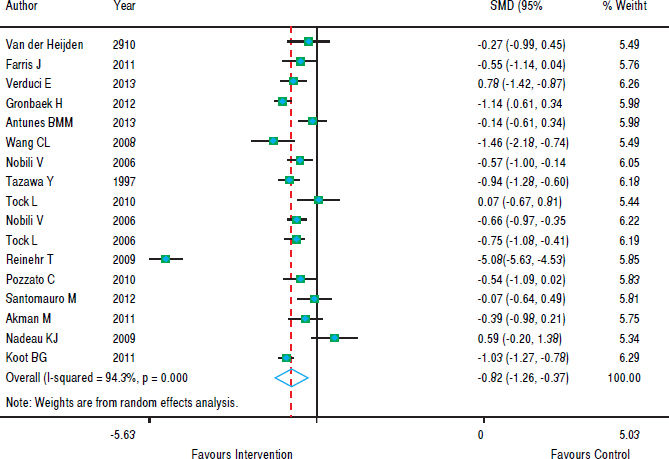
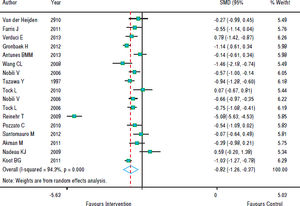
With regards to the effect of intervention on BMI, figure 2 shows that in eight studies the result was not significant and in nine studies there was a decrease in BMI z-score. As the heterogeneity between studies was high (p < 0.001), we used a random effects model to combine the studies and the standardized aggregate was -0.82 (95% CI: 1.26 to -0.37).
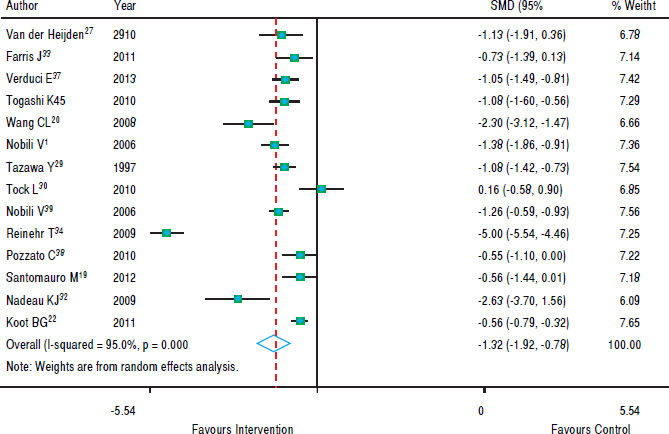
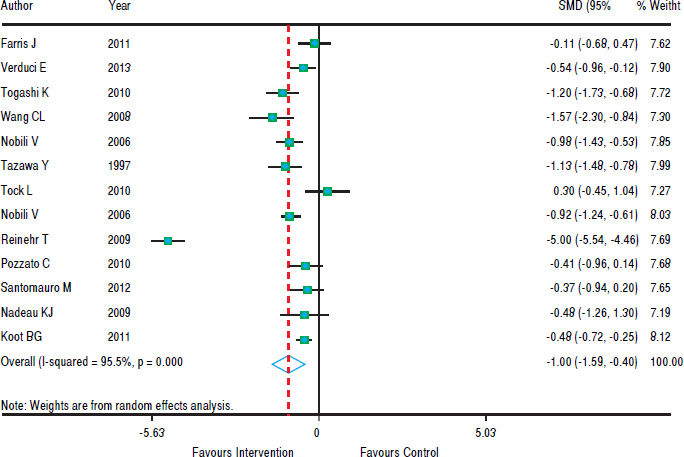
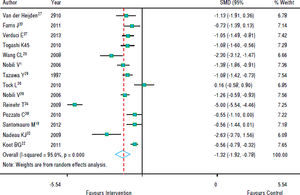
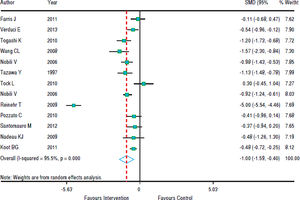
Figure 3 shows that in most studies there was a benefit from the intervention on ALT levels. The combined effect of using a random model was -1.35 (95% CI: -1.92 to -0.78), since the heterogeneity was increased (p < 0.001). In five studies, intervention showed no effect on AST levels. However, in eight studies there was a significant drop in AST. The combined effect was -1.00 standard deviation (95% CI: -1.59 to -0.40) (Figure 4).
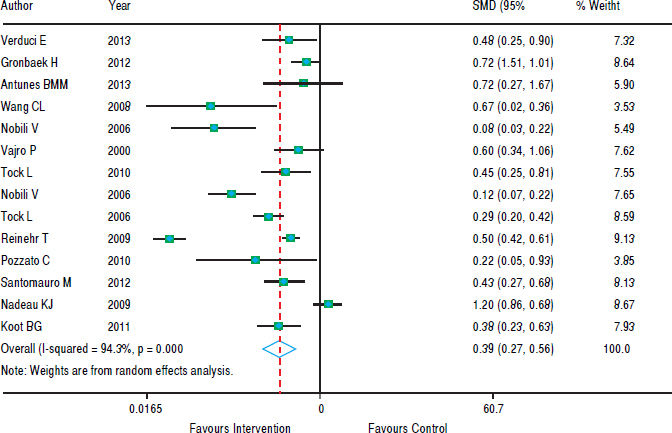
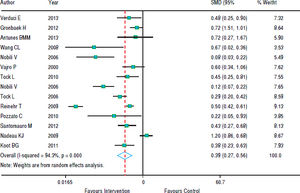
In figure 5, we note that the intervention was beneficial for hepatic steatosis in ten studies; but four studies showed no significant effect. The heterogeneity was high (p < 0.001) and the pooled effect was estimated using random - effects model. The risk of steatosis reduced by 61% after the intervention (RR grouped = 0.39; 95% CI: 0.27 to 0.56).
DiscussionNAFLD is the most prevalent pediatric liver disease in the world and has become a serious health problem, as well as a challenge for pediatricians who aim at treating but also preventing the evolution of this condition. Adult studies suggest that lifestyle changes can be beneficial for the treatment of NAFLD.10,25 The pediatric literature is characterized by small numbers of patients and heterogeneous interventions. This systematic review and meta-analysis shows that a balanced diet coupled with physical activity, improves BMI, aminotransferases and hepatic steatosis of children and adolescents with NAFLD, independent of puberty.19,35
Most patients with NAFLD are asymptomatic. NAFLD should be suspected in overweight / obese children and adolescents if their waist circumference is >> 95th percentile for age and sex.30
The outcome measures of this systematic review included changes in BMI and aminotransferase levels, as this is how patients are typically monitored in the clinical setting and these are the markers most often measured in pediatric interventional studies in the field of NAFLD. While they have both been found to correlate with disease severity, their sensitivity and specificity in determining steatosis is poor. BMI does not reflect body composition, and performs worse than waist circumference in predicting metabolic dysregulation, the hepatic manifestation of which is NAFLD.40 It has also been shown to miss over a quarter of children with excess adiposity.41 Aminotransferases have been shown to be inaccurate markers of steatosis when used for screening of NAFLD; ALT elevation above twice the upper limit of normal has a sensitivity 57% and specificity of 71% in this context.42
Magnetic resonance spectroscopy seems to be an imaging method quite promising, however, it still needs more studies.32
Given the invasive nature of liver biopsies, imaging is often used as an additional surrogate marker of steatosis. The available literature included in this systematic review reports predominantly on ultrasound-based estimates of hepatic steatosis. This is a significant limitation, as the positive predictive value of ultrasonography for the determination of steatosis ranges between 47-62%.43 Only a limited number of studies included the use of MRI, which albeit better than ultrasonography,43 its accuracy in pediatrics remains to be determined. An even fewer number of studies reported on the results of histology, which is considered the gold standard for the determination and quantification of hepatic steatosis in the context of NAFLD. The paucity of histological data also prevented the assessment of the impact of lifestyle interventions on hepatic fibrosis, which is the major determinant of long-term outcomes.44
The goal of treatment is the regression of steatosis/in-flammation and/or liver fibrosis. The decrease in ALT is commonly used as a marker of improvement for NAFLD.1
Most studies included in this systematic review and meta-analysis assessed the impact of both dietary and physical activity interventions. The study of Van der Heijden, et al.27 however, solely examined the impact of physical activity on the hepatic steatosis of adolescents. The results showed a significant reduction in hepatic steatosis mirrored by ALT changes, however, BMI z-scores did not change. The latter may be the result of the aforementioned limitations of BMI as a surrogate marker of obesity. Likewise, Ali, et al.28 assessed the impact of physical activity, which resulted in a non-significant reduction of BMI.
In the study of Farris, et al.,33 the intervention was performed in adolescents with physical activity associated to nutritional education. The results showed a statistically significant reduction in BMI and ALT levels. Similar results were found by Verduci, et al.,31 which also showed a significant reduction in steatosis.
Pozzato, et al.38 and Togashi, et al.45 observed a significant decrease in BMI, but it was not significant in reducing aminotransferases. In some studies, the authors tried to estimate how much weight reduction was needed for a significant result in the improvement of NAFLD to occur. These values ranged from 1 to 10% weight loss to obtain some benefit.29,31,34,39
Due to the association of this disease with overweight, the first step in the treatment has been to prevent obesity, stimulating lifestyle change through physical activity and a healthier diet.16–18 Physical exercise, even without weight loss, appears to decrease steatosis. Isolated exercise, without a calorie restriction, has not yet proven effective.19,22,23
In general, the studies seem to show that the decrease in weight is an important factor in reducing NAFLD markers. Even showing weight reduction variable values, there is a positive interference in the disease. The change in lifestyle seems, thus, to represent the first step in treating children and adolescents with NAFLD.
Lifestyle change with a healthier diet, gradual weight loss and increased physical activity seem to be the only effective treatment for this disease at present.6–14
This study has some limitations, such as the age of subjects, ranging from 6 to 18 years, justifying different pubertal stages of Tanner (pre-pubertal, pubertal and post-pubertal) and there may be some hormonal influence, although in many studies, this difference did not influence the results.19,37 The gold standard in the diagnosis of NAFLD is a liver biopsy, but it is difficult to carry out obese children considered “healthy” and without a proven effective treatment plan to justify the procedure. Thus, the vast majority of studies conducted diagnosis by abdominal ultrasonography, this being a standard that is operator dependent. Some of the studies were not randomized and some had no control group. None of the studies reported on long-term outcomes, specifically on what happens after lifestyle interventions are stopped. This is a key gap in the literature as NAFLD is a chronic condition with a constant impact on metabolic health. Finally, physical activity with respect to the time, intensity and duration differed between the included studies.
According to this systematic review and meta-analysis, lifestyle changes lead to improvement of NAFLD determined primarily via surrogate markers, even in patients who do not exhibit a significant weight reduction. More randomized controlled trials are needed to assess the impact of lifestyle changes on the histology of patients with NAFLD.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI: body mass index.
- •
MRI: magnetic resonance imaging.
- •
MRI: magnetic resonance imaging of the abdominal.
- •
MRS: magnetic resonance spectroscopy.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: steatosis or non - alcoholic steatohepatitis.